The Phenomenon of Quiescent Tomographically-Detected Macular Neovascularization

ABSTRACT
BACKGROUND
Macular neovascularization (MNV) is a pathophysiological precursor to local tissue destruction. Age-related macular degeneration is the predominant disease for MNV incidence. The spectrum of macular neovascular morphologies can be studied with optical coherence tomography angiography (OCTA) imaging and observed for vision-threatening activity. Some studies have found that MNV patterns with well-defined vessels are prevalent in active disease.9 In contrast, the ill-defined and long linear vessel patterns can illustrate a quiescent phenomenon signifying inactivity.
CASE REPORT
An 82-year-old man who developed a macular neovascular membrane was observed for two years without treatment because it was thought to be quiescent. OCTA was used to monitor the evolution of his MNV morphological changes, which ranged from quiescent, ill-defined neovascularization in the early stage to a well-defined vascular lesion later. Eventually, the MNV displayed spontaneous activity, provoking an exudative event, and anti-angiogenesis treatment was required.
CONCLUSION
The quiescent tomographically-detected macular neovascularization (QTMNV) phenomenon falls within the range of macular degeneration presentations and warrants close observation in the absence of exudative disease.
Keywords: Quiescent tomographically-detected macular neovascularization; macular degeneration; macular neovascularization, OCTA
INTRODUCTION
Macular neovascularization (MNV) is a complex physiological process facilitated by systemic antioxidant depletion, oxidative stress, and local melanocyte deprivation.[1] In the Consensus on Neovascular Age-Related Macular Degeneration Nomenclature (CONAN) Study Group classifications of macular neovascularization, the MNV types are classified by genesis location. Type 1 develops and remains in the choroid below the retinal pigment epithelium (RPE).[2] Type 2 forms in the choroid and grows through the RPE; type 3, previously known as retinal angiomatous proliferation (RAP), originates in the sensory retina and extends toward the RPE.2 Age-related macular degeneration (AMD) is the leading cause of MNV, and it occurs spontaneously in neovascular macular degeneration (nAMD) or when non-neovascular macular degeneration (nnAMD) progression causes MNV.[3] The potential for irreversible central vision loss caused by neovascular macular degeneration motivates the early detection and treatment of neovascular abnormalities.
Optical coherence tomography angiography (OCTA) effectively identifies macular neovascularization activity, location, and morphology. These morphologies can be described as the appearance of vessel shapes such as well-defined (“medusa” or “seafan”), long-linear (“filamentous” or “dead tree”), and ill-defined (indistinct or unidentifiable).[4] In addition to shape, the density of the vessels within the membrane can be classified as having a dense net or loose net configuration.[5] In conjunction with optical coherence tomography (OCT) imaging of structural macular pathology, OCTA helps confirm disease activity or inactivity and determine the indication for treatment.
CASE REPORT
An 82-year-old man without visual complaints was diagnosed with new signs of macular degeneration, specifically a shallow irregular retinal pigment epithelium elevation (SIRE) and macular neovascular membrane in his right eye, which was observed for two years. The frequency of follow-up ranged from one to six months, determined by lesion morphological changes, apparent stability, and macular degeneration progression. His medical history included diagnoses of complete atrioventricular block, atrial fibrillation, hypertension, cardiac pacemaker in situ, and chronic kidney disease. His systemic medications were lisinopril apixaban, aspirin, mirtazapine, and the AREDS 2 formula multivitamin. His ocular history included the diagnosis of a peripheral retinal tear in the right eye, which was treated with laser retinopexy.
His corrected Snellen visual acuity measured 20/25 in the right eye and 20/30 in the left eye throughout his eye exams, which was attributed to a cataract in each eye. His afferent and ocular motor exams were normal. Confrontation fields measured by finger counting were full in each eye. No Amsler grid defects were detected in either eye during the observation period. Biomicroscopy of the anterior segment showed healthy structures in each eye. His dilated fundus evaluation showed well-perfused optic nerves with a cup-to-disc ratio of 0.60 in the right eye and 0.35 in the left eye. The macula showed mild perifoveal retinal pigment epithelium atrophy with a small area of irregular elevation inferiorly in the right eye and minimal perifoveal retinal pigment epithelium atrophy in the left eye (Figure 1). There were no signs of drusen, hemorrhages, or areas of retinal edema in either eye.
Fundus photos and a series of macular images were captured with OCT and OCTA over two years. During the observation period, the left eye showed no pathology. The initial presentation of the MNV in the right eye was associated with a single perifoveal SIRE lesion (Figure 2). The incipient ill-defined neovascularization appeared in the outer RPE (Figure 3) and signaled the formation of a mixed type 1 and type 2 neovascular membrane in the central subfield zone.

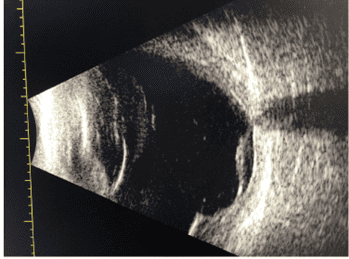
Figure 2 (left). OCT of the initial detection of a shallow irregular retinal pigment epithelium elevation in the right eye . Figure 3 (right) The initial detection of neovascular formation in the OCTA in the right eye
Six months after the recommended one-month follow-up, the appearance of the SIRE lesion was stable, and showed no signs of exudation (Figure 4). The OCTA imaging study detected expansion of the neovascularization from the sub-RPE to the outer retina, appearing in the outer retina to choriocapillaris (ORCC) en face projection (Figure 5). The lesion morphology was ill-defined with a loose net configuration. The lesion remained quiet during the first year of observation and developed a filamentous appearance (Figure 6), indicating the chronic lesion was now a quiescent tomographically-detected macular neovascularization (QTMNV) or “dead tree”.

Figure 4. OCT of a stable shallow irregular retinal pigment epithelium elevation in the right eye six months after initial presentation

Figure 5. At six months, the OCTA of an ill-defined quiescent tomographic macular neovascularization in the right eye. The surrounding hypointense halo is a sign of impaired blood flow

Figure 6. The OCTA of a well-defined tomographic quiescent macular neovascularization showing a loose net configuration with early filamentous morphology and low capillary density in the right eye more than one year after detection. The macular OCT cross-section of the shallow irregular retinal pigment epithelium elevation in the right eye appears in the lower frame
During year two, the QTMNV developed a dense net configuration (Figure 7), causing adjacent subretinal fluid and disorganization of the outer retinal structures (Figure 8). The neovascular activity resulted in nasal subfoveal elevation and incomplete outer retina atrophy (iORA) on OCT. The now-active tomographically-detected macula neovascularization was treated with a series of anti-vascular endothelial growth factor intravitreal injections for anti-angiogenesis mechanism. The macula appearance documented with wide-field color photos was unchanged (Figure 9).

Figure 7 (left). OCT of an inferior subfoveal retinal pigment epithelium elevation and adjacent subretinal fluid detected after two years of relative stability. Figure 8 (right) OCTA of hyperreflective neovascularization and dense net capillary activity.

Figure 9. Wide-field color fundus photos showing a stable macular appearance during the exudative AMD transformation
DISCUSSION
The OCTA program produces images of anatomical vessel networks in the retinal layers on a microvascular scale. Images captured with OCTA can detect neovascularization between the inner retina, outer retina, retinal pigment epithelium, and choroid (Table 1). Furthermore, manually shifting the projection boundaries can customize OCTA en face projections to capture any suspected areas of vasculature pathology.

Table 1. Correlation between select OCTA projection boundaries and imaged retinal layers or anatomical structures within each slab
Terminology describing the spectrum of neovascular morphologies imaged by OCTA has emerged, illustrating the qualitative characteristics defining disease activity and phase (Table 2). For example, the term “dense net” describes the proliferation of a neovascular network with dense capillary branching.[6] Further, dense branching patterns with vessel loops and anastomotic arcades have been correlated with active neovascular lesions.6 The term “loose net configuration” describes neovascularization with a low branching index.6 Loose net lesions have been associated with a longer disease duration than the acute, active dense net.6 The ill-defined indistinct and unidentifiable patterns show no detectable vessel pattern; moreover, the unidentifiable MNV pattern was associated with a significantly shorter disease duration.6 This system of describing MNV supplements the type 1-3 classification and may be applied for insight into disease behavior.
Further delineation describes well-defined MNV morphologies that include a medusa pattern, identified by the radiation of vessels from the center of the lesion, a sea fan shape, where the vessel network radiates from one side of the lesion, and a glomerulus ball-shaped vascular network. Ill-defined morphological vascular patterns have been detected in clinically active and inactive MNV cases.7,8 Long filamentous vessel patterns comprise the group of neovascular membranes that signal lesion inactivity and chronicity.8 The filamentous lesions showing a dead-tree or pruned-vascular-tree pattern with long linear vessels and absent capillary branching networks are seen in inactive or previously treated disease processes.[7]
A clear zone of absent vasculature that appears as a hypointense halo surrounding the neovascular lesion is a prevalent OCTA finding. These perilesional dark areas are surmised to develop from compensatory choriocapillaris activity triggered by reduced blood flow.[8] The correlation between perilesional halos surrounding quiescent lesions and the exudation risk was shown to be significant in one study.[9] However, the presence of a dense net pattern is a more significant predictor of active disease than the halo sign.10

Table 2. A survey of the optical coherence tomography angiography spectrum of macular neovascular morphologies and characteristics.9 The statistics shown for active and inactive MNV resulted from cross-sectional retrospective study data evaluating morphologic patterns of 63 choroidal neovascular membranes using OCTA.4 The investigation included 33 active lesions, of which 23 were well-defined, 8 were ill-defined, and 2 had long linear shapes. Of the 30 inactive MNV detected by OCTA, 3 were well-defined,14 were ill-defined, and 13 had long linear presentations.4
In the presence of SIRE lesions, quiescent or dead-tree macular neovascularization is considered a subclinical, nonexudative MNV.[10] It is also viewed as a protective measure against ischemia and often shows stability beyond six months.[11] Anti-vascular endothelial growth factor (VEGF) treatment is not recommended until definitive signs of exudative activity are associated with the neovascular membrane.13 In cases with OCTA-detected subclinical MNV, by 24 months, the risk of exudation was 13.6 times greater compared to eyes without subclinical MNV.13 Since, theoretically, anti-VEGF therapies used for subclinical neovascularization may accelerate the formation of atrophic macular disease, the recommendation for subclinical MNV without exudation is close follow-up without treatment.13 A natural reduction in intraocular VEGF levels facilitates the closure of peripheral capillaries and increased flow velocity, leading to arteriogenesis, the dilation, and the maturation of vessels at their interior.9 The results of arteriogenesis were characterized by large-diameter vessels, capillary network loss, and vessel anastomosis of a quiescent MNV.9
CONCLUSION
In this case of neovascular age-related macular degeneration, the spontaneous involution of the macular neovascular membrane was deemed a sign of inactivity and it was classified as a quiescent tomographically-detected macular neovascular membrane. The neovascularization remained inactive for two years without treatment. Predictors of lesion activity exist within the delineation of neovascular membrane morphologies as detected by OCTA. Structurally, macular neovascularization presenting as a dense or loose net within well- and ill-defined morphologies have substantiated statistical probabilities for active vs quiescent disease. As these variations of MNV are not absolute indicators of clinical activity or inactivity, the presentation in this case underscores the prudence of close observation.
Acknowledgement: Eric Eleff, MD, Northeast Ohio VA retinal specialist, provided consultation supporting the clinical decision for observation in this case
REFERENCES
- Vyawahare H, Shinde P. Age-Related Macular Degeneration: Epidemiology, pathophysiology, diagnosis, and treatment. Cureus. 2022 Sep 26;14(9):e29583
- Faatz H, Rothaus K, Ziegler M, Book M, et al. Vascular analysis of type 1, 2, and 3 macular neovascularization in age-related macular degeneration using swept-source optical coherence tomography angiography shows new insights into differences of pathologic vasculature and may lead to a more personalized understanding. Biomedicines. 2022;10(3):694
- Chew EY, Swaroop A. Age-related macular degeneration: from clinic to genes and back to patient management. 2021. Springer International Publishing
- Ahmed M, Syrine BM, Nadia BA, Anis M, et al. Optical coherence tomography angiography features of macular neovascularization in wet age-related macular degeneration: A cross sectional study. Ann Med Surg (Lond). 2021;70:102826
- Coscas GJ, Lupidi M, Coscas F, Cagini C, et al. Optical coherence tomography angiography versus multimodal imaging in assessing the activity of exudative age-related macular degeneration: a new diagnostic challenge. Retina. 2015;35(11):2019-28
- Sulzbacher F, Pollreisz A, Kaider A, Kickinger S, Sacu S, Schmidt-Erfurth U. Identification and clinical role of choroidal neovascularization characteristics based on optical coherence tomography angiography. Acta Ophthalmol. 2017;95:414-20
- Spaide R, Jaffe G, Sarraf D, Freund K, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: Consensus on Neovascular Age-Related Macular Degeneration
- Magdy M, Mahmound L, Hagar K. Imaging Choroidal neovascular membrane using en face swept-source optical coherence tomography angiography. Clin Ophthal. 2017;11:1859-69
- Solecki L, Loganadane P, Gauthier AS, Simonin M, et al. Predictive factors for exudation of quiescent choroidal neovessels detected by OCT angiography in the fellow eyes of eyes treated for neovascular age-related macular degeneration. Eye (Lond). 2021;35(2):644-50
- Notomi S, Shiose S, Fukuda Y, Mori K, et al. Characteristics of retinal pigment epithelium elevations preceding exudative age-related macular degeneration in Japanese. Ophthalmic Res. 2023;66 (1): 108–15
- Yang J, Zhang Q, Motulsky E, Thulliez M, et al. Two-year risk of exudation in eyes with nonexudative age-related macular degeneration and subclinical neovascularization detected with swept-source optical coherence tomography angiography. Am J Ophthalmol. 2019;208:1-11
Leslie R. Wilderson, OD, FAAO, Dipl AAO, graduated from the Nova Southeastern University College of Optometry, completed a Primary Eyecare Residency, and is board certified in medical optometry (ABCMO). Dr. Wilderson is an American Academy of Optometry Fellow, American Academy of Optometry Diplomate in the Comprehensive Eye Care Section, and Journal of Medical Optometry Editorial Board member. She is on the optometry staff at the Northeast Ohio VA and serves in the Air Force Reserve.