Navigating Choroidal Folds: A Comprehensive Approach for the Primary Care Optometrist
ABSTRACT
BACKGROUND
Choroidal folds are characterized by alternating grooves and ridges of the choroid, Bruch’s membrane, and retinal pigment epithelium (RPE), and they serve as a critical diagnostic sign in ocular disease. They often signal underlying pathologies such as chronic hypotony, orbital masses, or inflammation. Advanced imaging and a systematic differential diagnosis are essential to identify their etiology and in the prevention of vision- or life-threatening complications.
CASE REPORT
A 74-year-old male with advanced glaucoma and a history of bilateral trabeculectomies presented for routine eye care. Despite no visual complaints, examination revealed large superior blebs and marked hypotony (intraocular pressure 3 mmHg bilaterally). Dilated fundus exam demonstrated bilateral choroidal folds, confirmed by optical coherence tomography (OCT) showing choroidal undulations and intraretinal microcystic edema in the left macula. Given his surgical history and hypotony, a diagnosis of hypotony maculopathy was made. The glaucoma specialist consulted on the case recommended observation due to the patient’s asymptomatic status and prior unsuccessful interventions.
CONCLUSION
Choroidal folds are a key indicator of ocular or systemic pathology, with their typical horizontal orientation reflecting underlying anatomical and biomechanical constraints. This case underscores the importance of understanding the pathophysiology, utilizing advanced imaging (e.g. OCT), and engaging in interdisciplinary collaboration to guide management. Conservative management may be appropriate in asymptomatic, chronic cases, emphasizing individualized care to optimize patient outcomes.
Keywords: Choroidal folds, hypotony maculopathy, trabeculectomy, optical coherence tomography, glaucoma surgery
INTRODUCTION
Choroidal folds appear ophthalmoscopically as alternating dark and light striations in the posterior pole, reflecting undulations in the choroid, Bruch’s membrane, and RPE. First described by Nettleship in 1884 in a case of papilledema from an intracranial mass, these folds are now recognized as manifestations of a diverse range of etiologies, from benign idiopathic findings to vision- or life- threatening conditions such as ocular hypotony, idiopathic intracranial hypertension (IIH), or orbital tumors.1 Their clinical significance lies not only in their distinctive appearance but also in their role as a warning sign of underlying pathology, necessitating a thorough systemic and ocular evaluation. It was once assumed that choroidal folds are frequently idiopathic without an identifiable cause; however, recent studies indicate that a causative disorder can be identified in up to 85% of cases of choroidal folds with a thorough, comprehensive work-up, which includes numerous imaging studies and lumbar puncture.2 This high rate of associated causes underscores the importance of performing thorough workups when looking for an etiology whenever choroidal folds are observed.
The pathogenesis of choroidal folds is largely explained by biomechanical principles of tissue deformation. In 1989, Friberg proposed that compressive stress within the choroidal-Bruch’s membrane-RPE complex causes the tissue to buckle, analogous to a column collapsing under axial load.3 This model accounts for fold formation across numerous scenarios. For example, in ocular hypotony, reduced intraocular pressure (IOP) diminishes scleral support, leading to choroidal engorgement and inward compression of the choroid that produces folds. Many cases of choroidal folds demonstrate a horizontal orientation, which likely arises from anatomical constraints where anchoring by vortex veins and posterior ciliary arteries creates tension along the horizontal meridians and scleral rigidity limits the circumferential expansion, resulting in a favoring of horizontal buckling.3,4 Furthermore, choroidal folds frequently coexist with choroidal effusions or detachments, where fluid accumulates in the suprachoroidal space (as in hypotony), can induce folds or accentuate existing ones by increasing mechanical stress on the choroid. In turn, localized detachments (from inflammation, trauma, or hemorrhage) often exhibit folds at their margins due to focal tissue displacement during detachment and reattachment.
The spectrum of underlying etiology of choroidal folds includes tumors, hypotony, inflammation, neovascular membranes, retrobulbar masses, papilledema, and extraocular hardware, as described in the literature. Some of these etiologies are revealed by advanced ocular imaging, which can be critical for identifying and characterizing choroidal folds. Spectral-domain optical coherence tomography (SD-OCT) and enhanced-depth imaging (EDI-OCT) are crucial for detecting subtle structural changes, such as RPE undulations and choroidal thickening. Fluorescein angiography (FA) classically shows alternating hyperfluorescent streaks at fold crests (where the RPE is stretched and thinned) and hypofluorescent streaks at troughs (where the RPE is compressed).5 Indocyanine green angiography (ICGA) highlights choroidal vascular alterations, often revealing broader zones of choroidal hyperfluoresence corresponding to congestion beneath fold crests.5,6 B-scan ultrasonography and orbital imaging (CT/MRI) are invaluable to exclude posterior scleritis or an orbital mass as causes of folds.7 In summary, recognizing choroidal folds and correlating imaging findings with potential etiologies is an essential skill to ensure timely diagnosis of the underlying condition.
This case report presents one interesting case of bilateral choroidal folds secondary to chronic hypotony following trabeculectomy. By introducing this case, the discussion aims to enhance recognition of choroidal folds, as well as emphasizing their multifactorial etiologies.
CASE REPORT
A 74-year-old Caucasian male presented to the optometry clinic for routine eye care after having been lost to ophthalmology follow-up. He reported no visual complaints or changes. His ocular history was significant for advanced primary open-angle glaucoma managed with bilateral trabeculectomies over 30 years ago; the left eye had subsequent bleb needling and a failed autologous blood patch about 10 years ago. He had used glaucoma medications but discontinued them four years ago following an ophthalmologic evaluation that deemed his intraocular pressures (IOP) “too well controlled.” His medical history included well-managed hypertension, with no significant family ocular or systemic diseases.
On examination, best-corrected visual acuity was 20/50 OD and 20/150 OS, unchanged from prior records. Entrance testing, including confrontational visual fields and extraocular motility, was unremarkable. Goldmann applanation tonometry confirmed bilateral hypotony (3 mmHg OD and OS). Slit-lamp examination revealed large, avascular filtering blebs superiorly in both eyes, with no positive Seidel sign observed on fluorescein staining, ruling out active aqueous leakage and supporting overfiltration as the likely mechanism of hypotony.
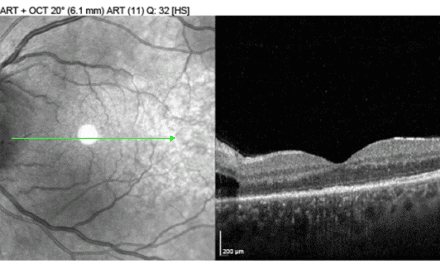
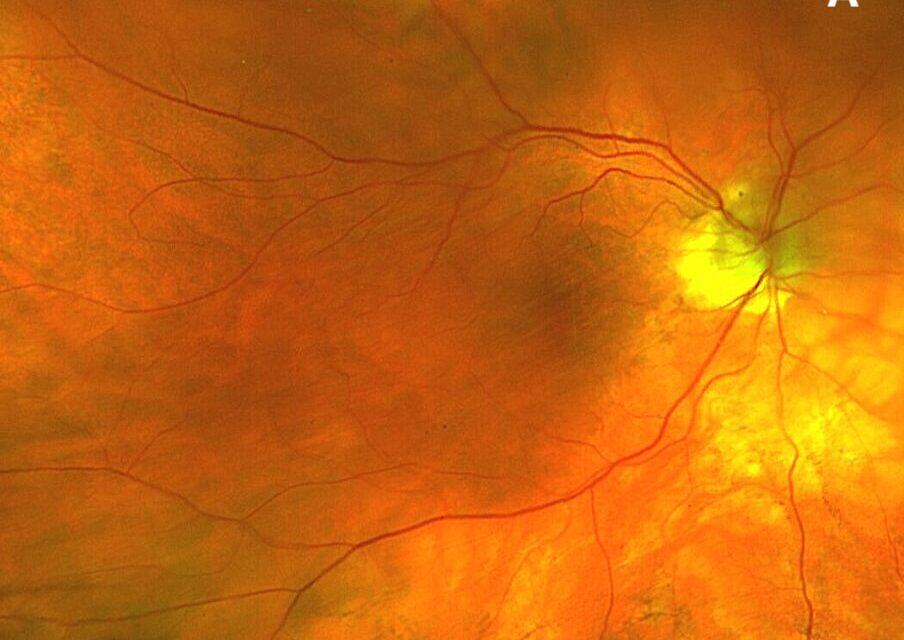
Dilated fundus examination demonstrated subtle choroidal folds in both eyes, most evident in the superior macular regions (Figure 1). OCT imaging revealed intraretinal microcystic edema in the left eye (Figure 2), a finding consistent with chronic hypotony maculopathy, which occurs due to prolonged low intraocular pressure altering retinal fluid dynamics. In addition, bilateral choroidal undulations were also evident on OCT (Figure 3) due to mechanical stress from scleral collapse.
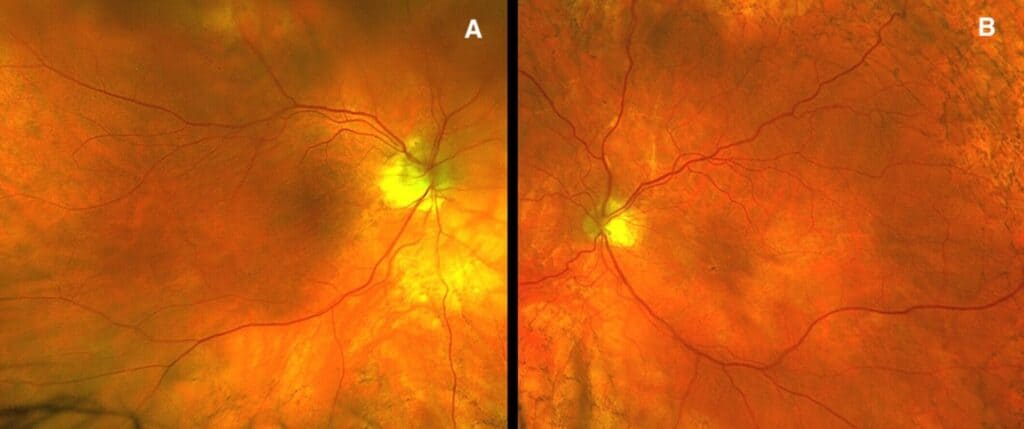
Figure 1. Fundus photo of right eye (A) and left eye (B) revealing subtle choroidal folds ST arcades.
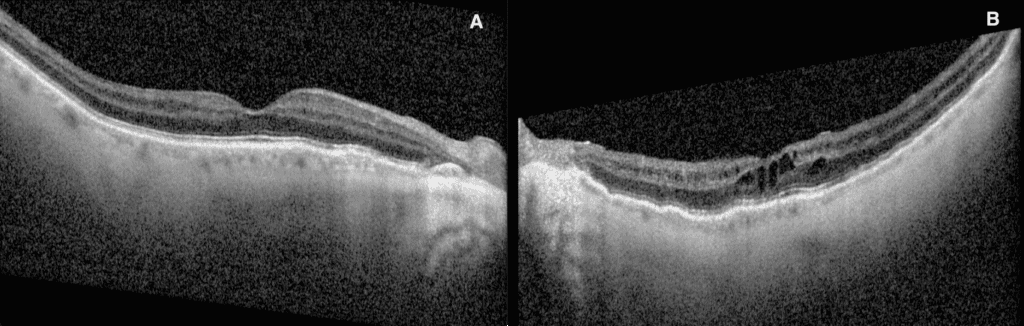
Figure 2. Macular OCT raster revealing no retinal edema OD (A) and intraretinal microcystic edema OS (B).
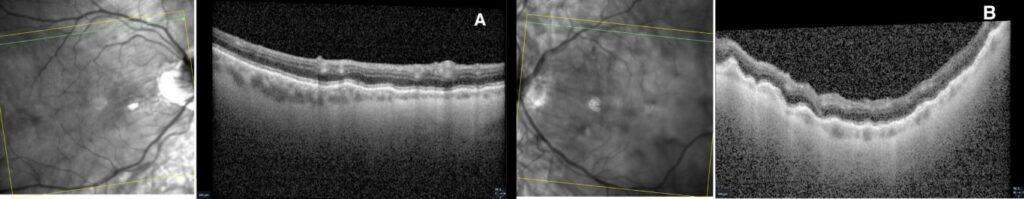
Figure 3. OCT raster through superior arcades showing bilateral choroidal folds in both right (A) and left (B) eyes.
Given the patient’s longstanding hypotony, complex glaucoma surgical history, and imaging studies, the patient was diagnosed with choroidal folds secondary to hypotony maculopathy. The patient was referred to a glaucoma specialist, who recommended observation due to his asymptomatic status, chronic hypotony, and prior unsuccessful interventions such as blood patching in the left eye.
DISCUSSION
Choroidal folds serve as a biomechanical fingerprint of ocular stress, and their presence warrants a careful investigation of potential causes. In this case, bilateral folds resulting from chronic hypotony following trabeculectomy provide an example of Friberg’s model of compressive stress, where prolonged reduced IOP (≤5 mmHg) induces scleral wall collapse and choroidal congestion, ultimately leading to the choroid-Bruch’s membrane-RPE complex to buckle upon itself.3 The predominantly horizontal orientation of the folds in the patient in this case is consistent with the typical pattern seen in hypotony and other causes, attributable to the sclera’s biomechanical properties and points of choroidal anchorage along the horizontal meridians.3,4 In addition, the relationship between choroidal folds and choroidal detachments is linked. In hypotony, fluid from within the suprachoroidal space may cause a choroidal detachment which also exacerbates any mechanical compression.8 Conversely, choroidal detachments secondary to inflammation or trauma often manifest with folds at their margins due to localized tissue displacement.9
Choroidal folds present a diagnostic challenge due to their association with diverse differential diagnoses, necessitating a systematic approach to pinpoint the underlying etiology. The cause was apparent in this case (long-standing post-surgical hypotony); however, clinicians must be able to adeptly exclude other ocular and systemic causes. Important ocular causes include choroidal or orbital tumors, such as choroidal melanomas or metastatic tumors originating from the breast or lung that can compress the globe, producing folds that may be curvilinear or radiating from the mass. These tumors often cause choroidal thickening and engorged choroidal vasculature, which can be visualized with EDI-OCT and confirmed on B-scan ultrasound.10,11 Any new unilateral choroidal folds warrant neuroimaging to rule out an orbital mass.12
In addition, posterior scleritis represents an uncommon but sight-threatening scleral inflammation that can account for a significant subset of choroidal fold cases. Posterior scleritis often presents with a classic ultrasonographic “T-sign” (fluid in Tenon’s space on B-scan) and may coexist with choroidal effusions and folds. It is frequently associated with systemic autoimmune conditions such as rheumatoid arthritis.13 Prompt recognition is critical, since folds due to posterior scleritis typically resolve with timely corticosteroid or immunosuppressive therapy with a corresponding improvement in vision.
Choroidal folds can also occur secondary to choroidal neovascularization (CNV) and fibrovascular tissue contraction, as seen in neovascular age-related macular degeneration or pathologic myopia. Gass first described radial chorioretinal folds as a telltale sign of CNV, caused by the subretinal scar tissue pulling on the choroid.2,14 Today, OCT angiography is a valuable tool in these cases; even when CNV is not evident on exam, OCTA can detect subclinical choroidal neovascular networks beneath chronic idiopathic folds. In patients with long-standing idiopathic choroidal folds, studies have found that a subset will eventually develop CNV, underscoring the need for periodic surveillance of these patients.
Papilledema due to IIH is another systemic condition that can produce choroidal folds. Typically, these folds are concentric around the swollen optic nerve head in the form of Paton’s lines. However, in a minority of IIH patients, choroidal folds may be present even in the absence of obvious optic disc edema.15 Choroidal folds in the absence of disc edema should raise suspicion for elevated intracranial pressure. Clinicians should have a low threshold to investigate for IIH (including neuroimaging and lumbar puncture) in patients with unexplained choroidal folds, especially if bilateral, to avoid missing this diagnosis.15,16
Other causes of orbital or globe compression can lead to folds as well, such as thyroid eye disease in Graves orbitopathy, where enlarged extraocular muscles and orbital congestion indent the globe and are detectable on CT or MRI.2 In addition, iatrogenic factors like scleral buckling procedures and large glaucoma drainage devices that physically deform the sclera and choroid can induce folds. A detailed surgical history is key in helping make the correct diagnosis. In eyes with shorter axial length (ie, nanophthalmos) or acquired hyperopia, a crowded posterior segment and relative scleral rigidity can result in spontaneous choroidal folds.2 In summary, the etiologies of choroidal folds range widely from intraocular to orbital to systemic causes.
Advanced imaging modalities greatly aid in the evaluation of choroidal folds and their underlying causes. As demonstrated in this case, OCT can visualize the undulating RPE and any associated retinal edema, allowing for documentation of fold extent and monitoring over time. Furthermore, SD-OCT visualizes choroidal thickening and RPE undulations, distinguishing choroidal from retinal folds. In addition to the use of SD-OCT to analyze the retinal layers, most OCT software also provide a near-infrared (IR) photo when acquiring OCT imaging. Choroidal folds often appear more prominently on near-IR imaging compared to standard color photos as the IR reflectance uses a longer wavelength that can penetrate to the level of the RPE and choroid, allowing for higher contrast to observe the subtle choroidal undulations. In practice, the alternating peaks and troughs of choroidal folds will stand out distinctly on IR images. When compared to normal fundus photos with the use of white light, the small pigmentary changes that occur by the structural changes in RPE and choroid will be masked by the overlying retinal details, making choroidal folds harder to appreciate.
Fluorescein angiography (FA) will reveal a classic pattern of alternating hyper and hypofluorescent bands corresponding to RPE thinning at fold peaks and compressing at troughs.5 ICGA can reveal choroidal vascular changes, such as delayed choroidal filling or hyperfluorescence, which is particularly helpful in cases like posterior scleritis or tumors where choroidal perfusion may be altered. B-scan ultrasound is indispensable for detecting posterior thickening or effusion and for identifying any scleral or orbital masses that might not be evident on fundus exam.10 If an orbital lesion is suspected, MRI or CT of the orbits should be performed promptly. In essence, multimodal imaging not only confirms the presence of choroidal folds but often provides clues to their origin, thereby directing further systemic or localized investigation.
Management of choroidal folds hinges on addressing the underlying etiology, and prognosis and outcome varies by cause. In hypotony-associated folds, timely IOP normalization via bleb revision or autologous blood patching often resolves folds and improves vision within weeks, particularly if initiated before macular atrophy develops.17 However, when hypotony is chronic (>6 months), folds and any associated maculopathy, such as RPE changes or cystoid edema, may only partially resolve, and visual acuity may not fully recover due to longstanding structural changes.8 In our case, given the duration of hypotony, observation was chosen over further surgical intervention, recognizing that the folds were longstanding and stable. Inflammatory etiologies, such as posterior scleritis, typically exhibit resolution of folds and visual improvement within weeks of initiating corticosteroids or immunosuppressants.16,18 Orbital tumors requiring excision or radiation demonstrate fold resolution in the majority of cases, with visual outcomes dependent on preoperative macular involvement. Idiopathic folds generally remain stable over time and do not require active treatment. Nonetheless, because a proportion of these patients can develop complications such as choroidal neovascularization, lifelong periodic monitoring is recommended.
In the presented case of chronic hypotony, no medical or surgical treatment was pursued based on the recommendations of the consulting glaucoma specialist. Topical therapies (such as corticosteroids to reduce inflammation or cycloplegic agents to reduce ciliary body movement) were considered but deemed unnecessary in the absence of active inflammation and given the longstanding nature of the hypotony. Surgical options, including bleb revisions or scleral grafting, were deferred due to the risks of further intervention outweighing potential benefits in a stable, asymptomatic eye that had adapted to low pressures over the years. The patient will be managed conservatively with close observation. While few non-surgical options exist for chronic hypotony-induced choroidal folds, individualized management remains key. In all cases of choroidal folds, careful follow-up is necessary to preserve vision, either by timely treatment of the underlying etiology or by managing new complications should they arise.
CONCLUSION
Choroidal folds are a critical diagnostic sign that should prompt clinicians to search for an underlying ocular or systemic cause. Their characteristic horizontal pattern reflects underlying biomechanical and anatomical influence, but it is the context in which they occur that guides their significance. In the case described, chronic post-surgical hypotony led to persistent folds without progressive vision loss, illustrating that in select scenarios, a conservative approach can be appropriate. Primary eye care providers should be adept at recognizing choroidal folds and initiating a targeted workup, including imaging and referrals, to rule out serious causes such as tumors, inflammation, or intracranial hypertension. Management is directed at the inciting cause, and the prognosis varies widely as some folds resolve completely with treatment of the cause, whereas long-standing folds may leave residual changes. Ultimately, a personalized, interdisciplinary strategy is essential. Regular monitoring with OCT, fundus exams, and necessary systemic evaluations ensures that any changes are detected early and managed promptly. By understanding the implications of choroidal folds, clinicians can prevent vision loss and identify systemic diseases that might otherwise go unnoticed, improving patient-centered outcomes.
REFERENCES
- Nettleship, E. (1884). Peculiar lines in the choroid in a case of postpapillitic atrophy. Trans Ophthalmol Soc UK, 4, 167-168.
- Agrawal M, Tripathy K. Choroidal Folds. [Updated 2023 Aug 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557772/
- Friberg TR. The etiology of choroidal folds. A biomechanical explanation. Graefes Arch Clin Exp Ophthalmol. 1989;227(5):459-464. doi:10.1007/BF02172899
- Friberg TR, Grove AS Jr. Choroidal folds and refractive errors associated with orbital tumors. An analysis. Arch Ophthalmol. 1983;101(4):598-603. doi:10.1001/archopht.1983.01040010598014
- Norton EW. A characteristic fluorescein angiographic pattern in choriodal folds. Proc R Soc Med. 1969;62(2):119-128. doi:10.1177/003591576906200204
- Haruyama M, Yuzawa M, Kawamura A, Yamazaki C, Matsumoto Y. Indocyanine green angiographic findings of chorioretinal folds. Jpn J Ophthalmol. 2001;45(3):293-300. doi:10.1016/s0021-5155(01)00323-9
- Atta HR, Byrne SF. The findings of standardized echography for choroidal folds. Arch Ophthalmol. 1988;106(9):1234-1241. doi:10.1001/archopht.1988.01060140394040
- Kokame GT, de Leon MD, Tanji T. Serous retinal detachment and cystoid macular edema in hypotony maculopathy. Am J Ophthalmol. 2001;131(3):384-386. doi:10.1016/s0002-9394(00)00794-7
- Augsburger JJ, Coats TD, Lauritzen K. Localized suprachoroidal hematomas. Ophthalmoscopic features, fluorescein angiography, and clinical course. Arch Ophthalmol. 1990;108(7):968-972. doi:10.1001/archopht.1990.01070090070042
- Shields CL, Manalac J, Das C, Ferguson K, Shields JA. Choroidal melanoma: clinical features, classification, and top 10 pseudomelanomas. Curr Opin Ophthalmol. 2014;25(3):177-185. doi:10.1097/ICU.0000000000000041
- Özkan B, Koçer ÇA, Altintaş Ö, et al. Choroidal changes observed with enhanced depth imaging optical coherence tomography in patients with mild Graves orbitopathy. Eye (Lond). 2016;30(7):917-924. doi:10.1038/eye.2016.93
- Jaworski A, Wolffsohn JS, Napper GA. Aetiology and management of choroidal folds. Clin Exp Optom. 1999;82(5):169-176. doi:10.1111/j.1444-0938.1999.tb06638.x
- Vermeirsch S, Testi I, Pavesio C. Choroidal involvement in non-infectious posterior scleritis. J Ophthalmic Inflamm Infect. 2021;11(1):41. Published 2021 Oct 27. doi:10.1186/s12348-021-00269-9
- Cohen SM, Gass JD. Bilateral radial chorioretinal folds. Int Ophthalmol. 1994;18(4):243-245. doi:10.1007/BF00951806
- Griebel SR, Kosmorsky GS. Choroidal folds associated with increased intracranial pressure. Am J Ophthalmol. 2000;129(4):513-516. doi:10.1016/s0002-9394(99)00412-2
- Lavinsky J, Lavinsky D, Lavinsky F, Frutuoso A. Acquired choroidal folds: a sign of idiopathic intracranial hypertension. Graefes Arch Clin Exp Ophthalmol. 2007;245(6):883-888. doi:10.1007/s00417-006-0455-7
- Costa VP, Arcieri ES. Hypotony maculopathy. Acta Ophthalmol Scand. 2007;85(6):586-597. doi:10.1111/j.1600-0420.2007.00910.x
- Grosso D, Borrelli E, Sacconi R, Bandello F, Querques G. Recognition, Diagnosis and Treatment of Chorioretinal Folds: Current Perspectives. Clin Ophthalmol. 2020;14:3403-3409. Published 2020 Oct 19. doi:10.2147/OPTH.S241002
Dr. Chan, a graduate of the University of California, Berkeley's optometry program, honed his expertise through a residency in primary care and ocular disease at the Portland VA Medical Center. His commitment to veterans' eye health led him to join the Jonathan M. Wainwright Medical Center in Walla Walla, Washington, before transitioning to his current role as a staff optometrist at the Salem Community Based Outpatient Clinic of the Portland VA Medical Center. Dr. Chan's comprehensive background and dedication to patient care make him a valuable contributor to the field of medical optometry.