Paradoxical Progression in Nerve Fiber Analysis from Peripapillary Retinoschisis
ABSTRACT
BACKGROUND
Peripapillary retinoschisis (PPRS) is a splitting of the peripapillary nerve fiber layer, ganglion cell layer, inner plexiform layer, inner nuclear layer, outer plexiform layer, and outer nuclear layer. PPRS occurs in about 6% of all primary open-angle glaucoma (POAG) and glaucoma suspects, with a higher prevalence in advanced POAG cases. While the mechanism of action is unknown, it has been postulated to be related to mechanical forces on the optic nerve head. It is essential to closely monitor changes in the retinal nerve fiber layers in these patients, as they may be confused with glaucomatous progression.
CASE REPORT
A 78-year-old Caucasian male presented for follow-up imaging for glaucoma. He reported good compliance with his glaucoma drop therapy: latanoprost 0.005% ophthalmic solution one drop at night in both eyes, timolol 0.5% gel-forming solution one drop in the morning in each eye, and brimonidine 0.1% ophthalmic solution twice a day in the left eye. He denied any changes in his vision. He also had an ocular history of peripapillary retinoschisis in the left eye, mild hypertensive retinopathy, and cataracts in both eyes. His best corrected visual acuity was 20/20 in each eye. Humphrey visual field (HVF) testing at the exam in question revealed no glaucomatous changes in the right or left eye, which was an improvement from past testing, which found an early superior arcuate defect in the left eye. Upon reviewing all prior optical coherence tomography (OCT) scans, it was determined that the superior arcuate defect may have developed during the active phase of PPRS, and resolved as the schisis resolved. These results underscore the importance and value of OCT in managing glaucoma.
CONCLUSION
While quantifying the nerve fiber with OCT technology is an invaluable tool, it is important to qualitatively view peripapillary OCTs to ensure the integrity of the retina. PPRS can cause fluctuations in retinal nerve fiber layer thickness; thus, the spontaneous resolution of PPRS can mimic glaucoma progression. Though PPRS can be associated with glaucoma, not all PPRS presentations represent glaucomatous progression. Diagnostic tests, including OCT retinal nerve fiber layer and HVF, along with clinical exam findings, can help distinguish PPRS evolution from true glaucomatous progression.
Keywords: peripapillary retinoschisis, retina, glaucoma, optical coherence tomography, multi-modal imaging
INTRODUCTION
Peripapillary retinoschisis (PPRS) forms due to the splitting of the peripapillary nerve fiber layer, ganglion cell layer, inner plexiform layer, inner nuclear layer, outer plexiform layer, and outer nuclear layer.1 While the mechanism of action is unknown, it has been postulated to be related to mechanical forces on the optic nerve head.2 The presence of PPRS is accompanied by the transient thickening of the retinal nerve fiber layer (RNFL) as seen on optical coherence tomography (OCT) with subsequent RNFL thinning upon resolution. This thinning seen on the OCT RNFL scan can mimic glaucomatous progression. Though PPRS may occur in normal eyes, it occurs in 6% of all primary open-angle glaucoma (POAG) and glaucoma suspects with a higher prevalence in advanced POAG cases.2 As a result, it is important to monitor changes in the RNFL thickness closely as it may indicate true glaucoma progression. This case exemplifies the proper use of multi-modal imaging to elucidate retinal changes from glaucomatous progression.
CASE REPORT
A 78-year-old Caucasian male presented to the eye clinic for a follow-up exam and imaging. He had a pertinent ocular history of mild primary open-angle glaucoma (POAG) in the right eye and moderate POAG in the left eye, peripapillary retinoschisis in the left eye, mild hypertensive retinopathy, and cataracts in both eyes. He reported good compliance with his glaucoma drop therapy: latanoprost 0.005% ophthalmic solution one drop at bedtime in both eyes, timolol 0.5% gel-forming solution one drop in the morning in both eyes, and brimonidine 0.1% ophthalmic solution one drop twice a day in the left eye. He denied any changes in his vision.
His best corrected visual acuity was 20/20 in both eyes. Entrance testing, including pupils, extraocular muscle testing, and confrontation visual fields, was unremarkable. Anterior segment evaluation revealed trace blepharitis, concretions on his lower palpebral conjunctiva, and an instant tear break-up time in both eyes. His intraocular pressure (IOP) taken with non-contact tonometry at 9:26 a.m. was 17 mmHg in the right eye and 15 mmHg in the left eye, both of which was within his target IOP of 18 mmHg or less in the right eye and 15 mmHg or less in the left eye, in the setting of thinner-than-average pachymetry measurements (534 μm in the right eye and 541 μm in the left eye).
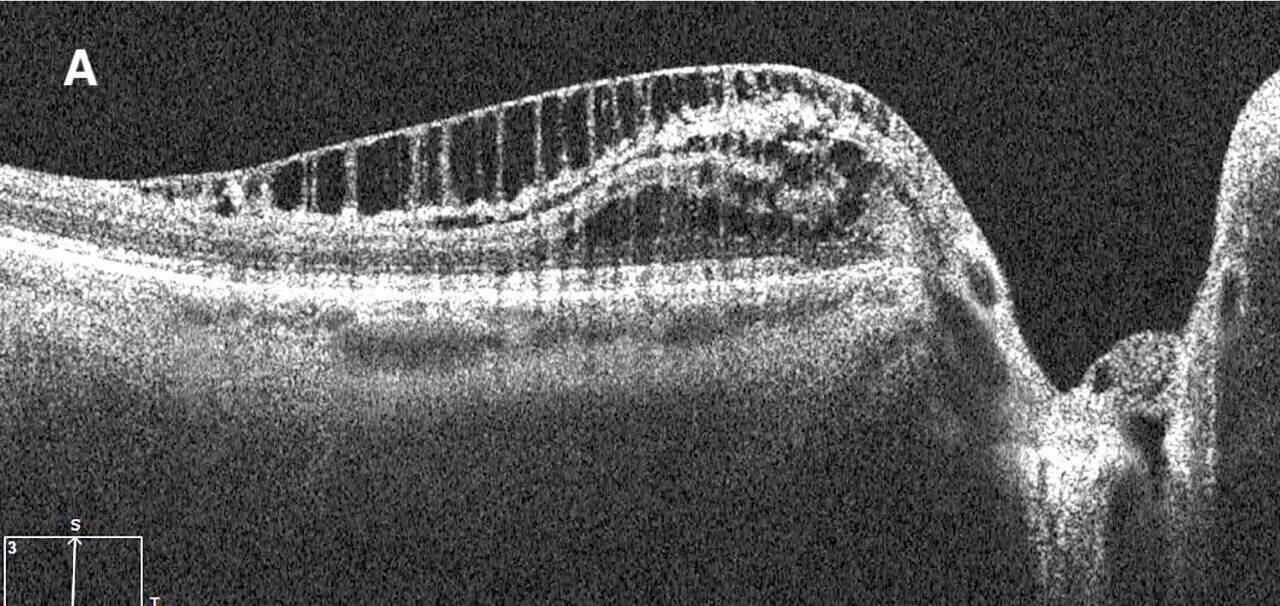
Dilated posterior segment evaluation revealed a cup-to-disc ratio of 0.70 round in the right eye and 0.75 round in the left eye, with good color and rim tissue, and without hemorrhage in both eyes. RNFL OCT was flagged for borderline thinning inferiorly in the right eye. Additionally, borderline RNFL thinning was evident temporally and inferiorly in the left eye (Figure 1a,b). The guided progression analysis showed no progression in the right eye and progression inferiorly in the left eye. When each scan was compared, the RNFL average thickness and quadrant measurements in the left eye remained the same before the onset of PPRS and following its resolution (Figure 2). An OCT scan of the left optic nerve displayed a resolved PPRS inferiorly and a newly noted PPRS superiorly (Figure 3).
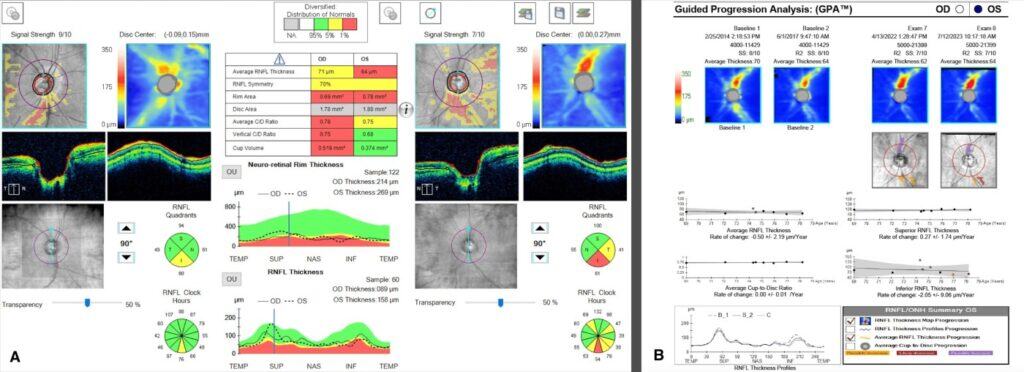
Figure 1. OCT-RNFL showed borderline thinning inferiorly in the right eye and borderline thinning temporally with thinning inferiorly in the left eye (A). Guided Progression Analysis (GPA) shows progression inferiorly in the left eye. (B)
It is important to note that the patient was determined to have progressed from mild POAG to moderate POAG four years prior, when the early superior arcuate defect in the left eye was detected on HVF SITA Fast 24-2 testing. However, OCT RNFL quadrant measurements from that time frame show RNFL thickening inferiorly rather than the typical thinning that would be expected to occur in glaucoma progression (Figure 2A and 2B). In addition, HVF SITA Fast 24-2 testing during the most recent exam revealed that the superior arcuate defect in the left eye was non-repeatable (Figures 4A, B). Therefore, the superior arcuate defect was most likely associated with the active phase of the PPRS. The patient was scheduled for a repeat HVF 24-2 in three months and advised to maintain his current glaucoma drop regimen.
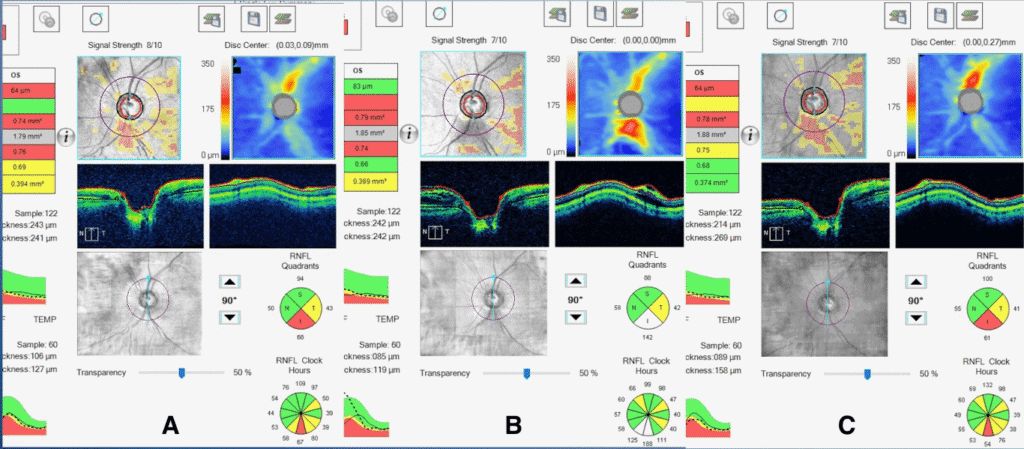
Figure 2. A) OCT RNFL of the OS eye six years prior to the current exam (and before the onset of PPRS). B) OCT RNFL of the OS four years prior to the current exam (when the PPRS was first documented). C) OCT RNFL of the OS taken at the current exam (after the resolution of PPRS).
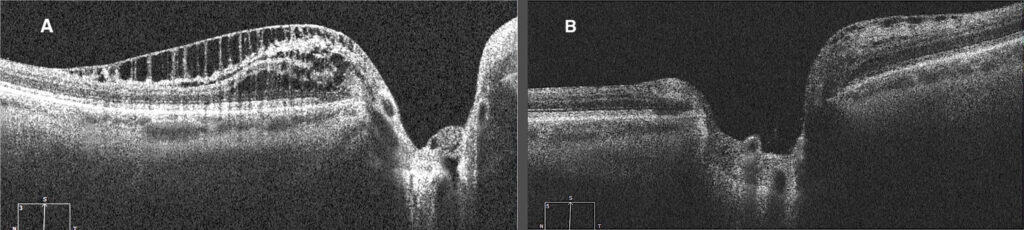
Figure 3. A) OCT of the OS of PPRS inferior to the ONH at onset (four years prior to the current exam). B) OCT of the OS of resolved inferior PPRS with a new onset PPRS superior to the ONH (taken during the current exam).
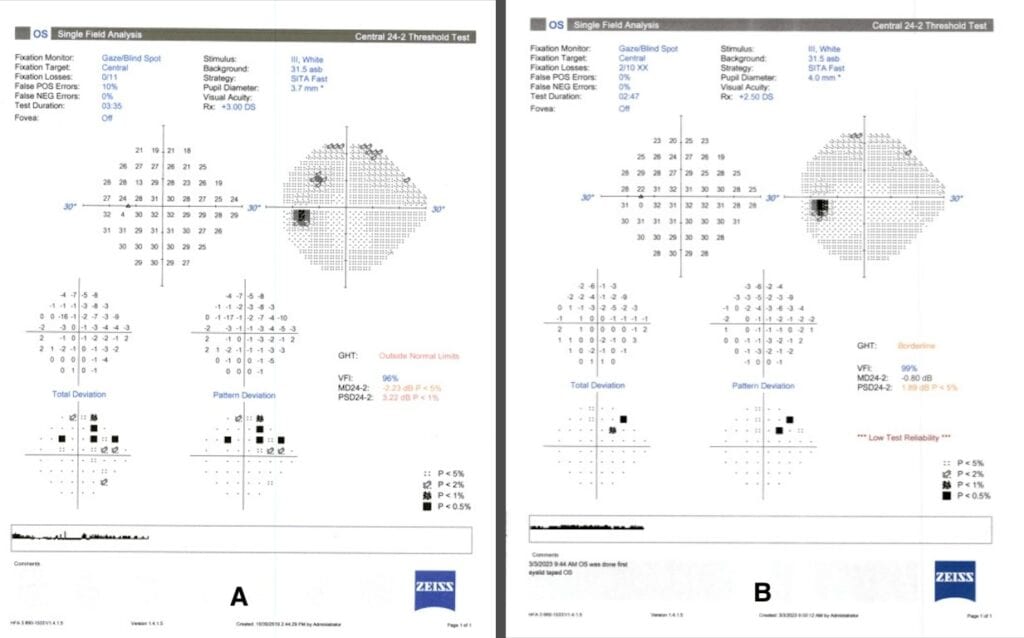
Figure 4. A) HVF Sita Fast 24-2, taken four years prior to the current exam, with an early superior arcuate defect in the left eye. B) HVF Sita Fast 24-2, taken during the current exam shows the early superior arcuate defect was much improved.
DISCUSSION
Retinoschisis is the abnormal splitting of the sensory retina. Before the development of OCT imaging, PPRS presentation was mainly associated with X-linked retinoschisis, optic disc abnormalities, and degenerative myopia.2 However, following the increased use of OCT imaging, recent studies have documented the presence of PPRS in glaucomatous eyes.3
PPRS will present on OCT scans as a schisis cavity with hyper-reflective bridging structures that cast shadows on the posterior retina.2 Due to the occurrence of PPRS in various types of glaucoma, including open-angle glaucoma, normal tension glaucoma, and intermittent acute angle closure glaucoma, it has been proposed that the mechanism of PRRS in these eyes is likely due to different etiologies. Though the true mechanism behind the formation of PPRS is largely unknown, some proposed mechanisms include a chronic increase in IOP, vitreopapillary traction, and Müller cell dysfunction.2,4 PPRS associated with bridging structures generally suggest Müller cell dysfunction associated with glaucoma.2 However, it is unclear whether the Müller cell dysfunction results in glaucomatous PPRS formation or if it is a response to mechanical stress on the retinal layers.1
A cross-sectional examination conducted in 2021 used spectral-domain OCT (SD-OCT) and Bruch’s membrane opening (BMO) as the anatomical boundary around the optic nerve head (ONH) to determine the pathogenesis of PPRS.1 If the proximal edge of the PPRS is behind the BMO, then the layers of the retina which would likely be affected are the ganglion cell layer, inner plexiform layer, and retinal nerve fiber layer.1 PPRS can also present with vitreous traction followed by a posterior vitreous detachment, thus the proposed etiology of the PPRS in these cases is mechanical vitreous traction on the retina.1 If the proximal edge of the PPRS is past the BMO, it will likely affect the inner nuclear layer, outer plexiform layer, and outer nuclear layer.1 The proposed etiology of this type of PPRS is secondary to the invasion of liquified vitreous through compromised Bruch’s membrane to the underlying retinal tissue.1 It is suggested that the micro-holes found in glaucomatous eyes due to Müller cell dysfunction leads to vitreous fluid invasion to the RNFL resulting in the formation of PPRS.1,2 The OCT of the PPRS found in the patient’s left eye, shows that the proximal edge is past the BMO (Figure 3A). This suggests that the PPRS formed as a result of the vitreous leaking through Bruch’s membrane and into the retinal tissue below it.
Though PPRS can occur in normal eyes, studies have shown that PPRS occurs significantly more often in glaucomatous eyes.5 It is found in about 6% of all primary open-angle glaucoma (POAG) and glaucoma suspects, with a higher prevalence in advanced POAG cases.2,5 These patients tend to present without symptoms, as it often does not expand to form macular retinoschisis.6 However, there are two reported cases with PPRS and macular retinoschisis where both individuals presented with narrow angles and elevated IOP.7 Most PPRS cases have been identified through incidental findings during OCT imaging, where there will be an associated transient increase in RNFL thickness.8
It is important to note that multiple PPRS events can occur in the same eye, at different points in time or in different locations.8 The most common location of the PPRS varies from study to study. A study by Ji-Ah Kim et al found that PPRS is more common in the inferior temporal quadrant while a study by Serife Bayraktar et al stated that they are more commonly found in the superior quadrant.5,9 Therefore, it is strongly recommended that high-definition scans and OCT imaging be performed for all glaucomatous patients. Once discovered, PPRS does not require treatment as it tends to be self-limited. Most cases resolve in 2-5 years, while some persist longer.2 In cases that present with a macular schisis, retinal detachment, progressive glaucomatous change, or serous retinal detachment, treatment should be initiated. Treatment includes but is not limited to IOP-lowering drop therapy, glaucoma filtration surgery, laser, pars plana vitrectomy, and trabeculectomy.4,10
Though PPRS typically does not require any treatment, it is essential to monitor peripapillary retinoschisis due to the resultant changes in RNFL thickness. At onset, RNFL thickens, leading to falsely elevated thickness. Resolution results in thinning and must be differentiated from glaucomatous progression. A high-definition scan should be considered for a proper diagnosis of PPRS. Following diagnosis, it is crucial to closely monitor changes in the retinal nerve fiber layers, as these changes can indicate either peripapillary retinoschisis or true glaucomatous progression. Viewing various diagnostic tests, such as OCT RNFL and HVF, along with clinical exam findings, can help distinguish the cause of change to the RNFL thickness. During the active stage, retinal findings from PPRS can cause an increase or improvement in the RNFL, leading to a false negative result. As the retina heals, there is often a decrease in RNFL thickness, mimicking progression.5
To determine if changes in the RNFL are due to glaucomatous etiology, the eye-care provider should look at data from multi-modal imaging. If PPRS presents without glaucoma, there should be no difference in IOP measurements, average RNFL thickness, and visual field indices before the formation and following the resolution of PPRS.8 Glaucomatous eyes with PPRS tend to exhibit higher IOP measurements, worse visual field MD values on the HVF 24-2, and rapidly worsening global average RNFL thickness values.2,6 High-definition imaging will provide a qualitative view of the tissue around the optic nerve to help identify the proximal edge of the PPRS and the affected retinal layer.
The PPRS seen in this case presented with hyperreflective bridging structures with shadows casting over the underlying retina, which suggests Müller cell involvement with possible glaucomatous associations. It is still unknown whether Müller cell dysfunction results in PPRS formation in glaucomatous eyes or presents as a response to changes occurring at the ONH from glaucomatous mechanical stress.2 Nevertheless, given the stability in average RNFL thickness measurements and of the visual field MD before the formation of the PPRS and following its resolution in this case, the retinal changes seen are likely not an indication of glaucomatous progression. Therefore, careful monitoring with multi-modal imaging will be considered without modifying the existing glaucoma regimen.
CONCLUSION
Although PPRS can be observed in normal eyes, the development of OCT has revealed that PPRS is also associated with open-angle glaucoma, normal-tension glaucoma, and intermittent acute angle-closure glaucoma. Patients with PPRS are usually asymptomatic, as it does not typically involve the macular region. This is why, in most cases, PPRS does not require any treatment and is largely self-limiting. However, on rare occasions, PPRS can be associated with serous retinal detachments, retinal detachments and progressive glaucomatous change. In these instances, glaucoma drop therapy, glaucoma filtration surgery, retinopexy, pars plana vitrectomy, and trabeculectomy may be considered. PPRS can be noted and monitored with OCT RNFL scans. The onset of PPRS will present as RNFL thickening followed by RNFL thinning upon resolution. These changes in RNFL thickness should be monitored closely because they can either indicate new or resolving PPRS or true glaucoma progression. Therefore, it is important to qualitatively view peripapillary OCTs along with other imaging modalities to distinguish the etiology behind the change in RNFL thickness and PPRS formation. By doing so, eyecare providers can better understand the patient’s disease process and determine the best course of action to manage it.
REFERENCES
- Nishijima R, Ogawa S, Nishijima E, Itoh Y, Yoshikawa K, Nakano T. Factors determining the morphology of peripapillary retinoschisis. Clin Ophthalmol. 2021;15:1293-1300.
- Fortune B, Ma KN, Gardiner SK, Demirel S, Mansberger SL. Peripapillary retinoschisis in glaucoma: Association with progression and OCT signs of Müller cell involvement. Invest Ophthalmol Vis Sci. 2018;59(7):2818-2827.
- Fortune B. Pulling and tugging on the retina: Mechanical impact of glaucoma beyond the optic nerve head. Invest Ophthalmol Vis Sci. 2019;60(1):26-35.
- Young B, Munoz M, Minino Soto E. Peripapillary retinoschisis in a patient with severe primary open angle glaucoma. JoMO. 2024;1(1):1-7.
- Kim JA, Lee EJ, Kim TW. Impact of peripapillary retinoschisis on visual field test results in glaucomatous eyes. Br J Ophthalmol. 2023;107(9):1281-5.
- Lee EJ, Kim TW, Kim M, Choi YJ. Peripapillary retinoschisis in glaucomatous eyes. PLoS One. 2014;9(2).
- Kahook MY, Noecker RJ, Ishikawa H, Wollstein G, Kagemann L, Wojkowski M. et al. Peripapillary schisis in glaucoma patients with narrow angles and increased intraocular pressure. Am J Ophthalmol. 2007 Apr; 143(4):697-9.
- Hwang YH, Kim YY, Kim HK, Sohn YH. Effect of peripapillary retinoschisis on retinal nerve fibre layer thickness measurement in glaucomatous eyes. Br J Ophthalmol. 2014;98(5):669-674.
- Bayraktar S, Cebeci Z, Kabaalioglu M, Ciloglu S, Kir N, Izgi B. Peripapillary Retinoschisis in Glaucoma Patients. J Ophthalmol. 2016;2016:1612720.
- Senthilkumar V, Mishra C, Kannan N, Raj P. Peripapillary and macular retinoschisis – A vision-threatening sequelae of advanced glaucomatous cupping. Indian J Ophthalmol. 2021;69(12):3552-8.