Benign Yellow Dot Maculopathy
ABSTRACT
BACKGROUND
Benign Yellow Dot Maculopathy is a novel retinal phenomenon first described in 2017 by Dev Borman et al. This finding represents a non-progressive condition in asymptomatic patients with normal visual function. To date, only 47 cases have been reported in seven published articles. The purpose of this case report is to describe detailed clinical findings of one patient with this new macular phenotype and to raise awareness of this condition.
CASE REPORT
A 31-year-old white male Veteran, asymptomatic with good vision, was discovered to have apparent yellow crystals scattered within the maculae of both eyes. Fundus autofluorescence images revealed hyper-autofluorescent spots consistent with the fundoscopic findings. No lesions were visible on OCT scan or OCT angiography. Electrophysiology tests showed normal inner and outer retinal functions. Following a comprehensive history assessment that ruled out toxic maculopathies, Late-onset Foveal-Sparing Stargardt Disease, Multifocal Pattern Dystrophy Simulating Stargardt Disease, Familial Dominant Drusen, Age-Related Macular Degeneration, Bietti’s Crystalline Dystrophy, North Carolina Macular Dystrophy, and Type 2 Telangiectasia. The patient received a diagnosis of Benign Yellow Dot Maculopathy.
CONCLUSION
Benign Yellow Dot Maculopathy represents a unique phenotype with limited coverage in existing literature. The lack of reports on this condition might be linked to its asymptomatic and generally benign nature and the general lack of clinical knowledge of the disease.
Keywords: Benign Yellow Dot Maculopathy, novel macular phenotype
INTRODUCTION
Many macular diseases could present with yellow macular dots. The differential diagnosis can be challenging due to a wide variety of etiologies, including toxic, genetic, degenerative, idiopathic, and iatrogenic causes. The majority of these cases are progressive, exhibiting typical systemic or ocular findings and are associated with reduced best-corrected visual acuity. In 2017, Dev Borman et al. introduced a new macular phenotype called Benign Yellow Dot Maculopathy. It is a non-progressive condition characterized by discrete yellow dots surrounding the fovea, more often bilaterally, in asymptomatic patients with normal visual function.1 At the time of this report, only 47 prior cases have been detailed in seven published articles. The purpose of this case report is to describe one patient with this new macular phenotype, providing detailed clinic findings and raising awareness of this condition.
CASE REPORT
A 31-year-old white male Veteran was referred by his primary care provider for a routine eye exam. The patient was new to the eye clinic and had not undergone a complete eye exam since a laser-assisted in-situ keratomileusis (LASIK) procedure to only his right eye seven years prior. The previous eye exam had been unremarkable. The patient reported that his vision was adequate at both distance and near without correction. He denied experiencing symptoms such as flashes, floaters, diplopia, or loss of vision. In his ocular history, he mentioned having metal in his eye as a teenager, although he was unsure which eye was affected. His past medical history included a history of alcohol abuse, Breast Cancer gene 1 (BRCA 1) mutation, right knee pain, obstructive sleep apnea and chronic lower back pain. The patient was not taking any medications and had no known drug allergies. The family’s ocular history revealed early glaucoma in his mother, with no reported instances of macular degeneration or blindness. Two siblings were noted to have normal functional vision, although the patient expressed uncertainty regarding their ocular health status. His father had passed away so information about paternal ocular health remained unknown.
Clinical Findings
The patient’s best-corrected visual acuity was 20/20 in each eye. The entrance exams, including cover test, extraocular muscles (EOMs), counting finger fields, pupils, and color vision were unremarkable for each eye. Anterior segment findings were unremarkable, except for a pinguecula in both eyes and a LASIK scar in the right eye only. Intraocular pressures (IOPs) were normal and symmetric. Posterior segment findings showed pinpoint, bright yellow, discrete dots scattered throughout the macular region between the arcades in both eyes. The mid- and far-periphery of the retina appeared normal without the yellow dots. The crystalline lens, vessels, and optic nerve heads were otherwise unremarkable.
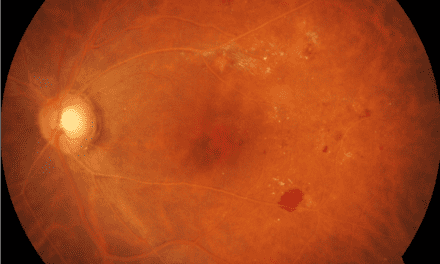
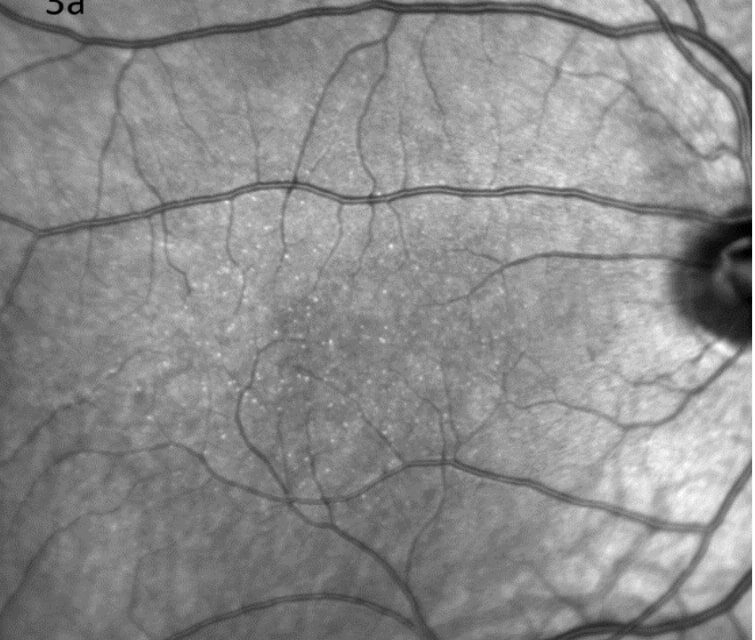
Color fundus images showed tiny discrete yellow dots in the macular region which appeared slightly duller compared to the slit lamp fundoscopic exam (Figure 1). Fundus AutoFluorescence (FAF) blue images showed tiny hyper-autofluorescent spots corresponding to the yellow dots (Figure 2). The yellow dots appeared hyper-reflective on near-infrared (near-IR) imaging (Figure 3). The dots were more enhanced on near-infrared images compared to FAF blue images. However, OCT scans were normal without retinal lesions (Figure 4). OCT angiography (OCT-A) was unremarkable for both eyes. Pattern electroretinography (pERG) and multifocal electroretinography (mfERG) were all within the normal range, signifying normal function of both the central outer retinal (cones), and the inner retina (ganglion cells) (Figure 5 & 6).
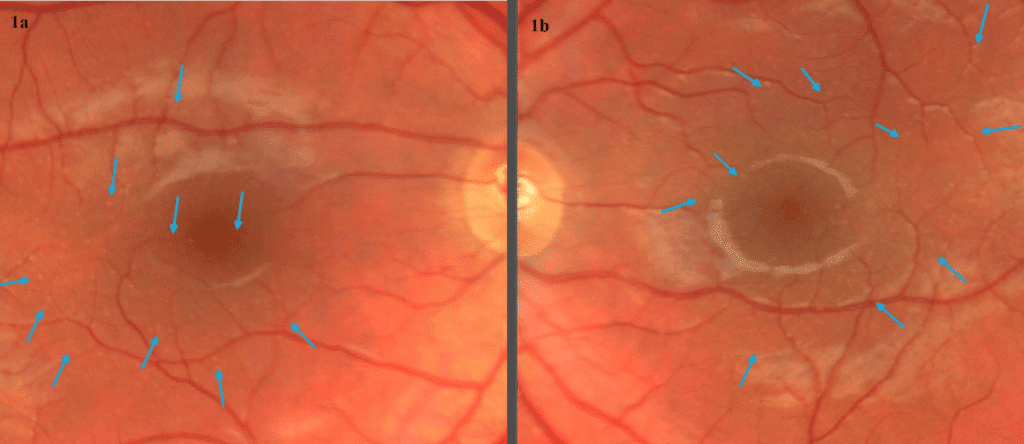
Figure 1. Color fundus photography of right (a) and left (b) eyes showed tiny discrete yellow dots scattered in the macular region. The dots appeared duller on fundus photo compared to the bright yellow presentation on slit lamp fundoscopic exam.
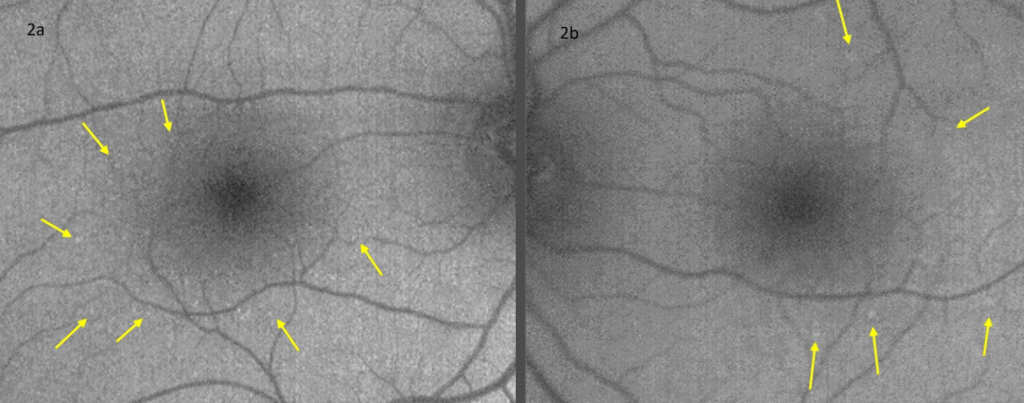
Figure 2. Fundus autofluorescence blue photography of right (a) and left (b) eyes revealed hyper-autofluorescent dots in both maculae.
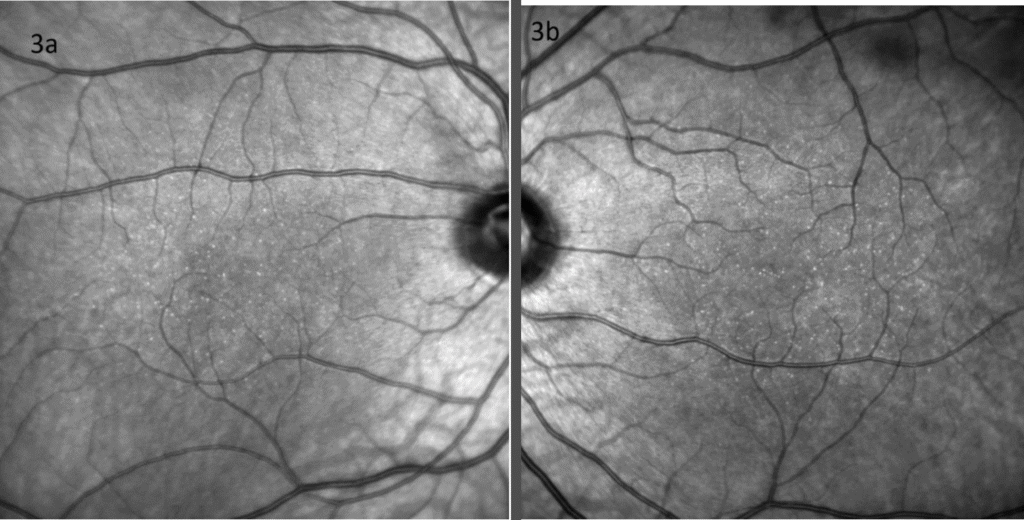
Figure 3. The yellow dots appeared hyper-reflective on near-infrared imaging of right (a) and left (b) eyes.
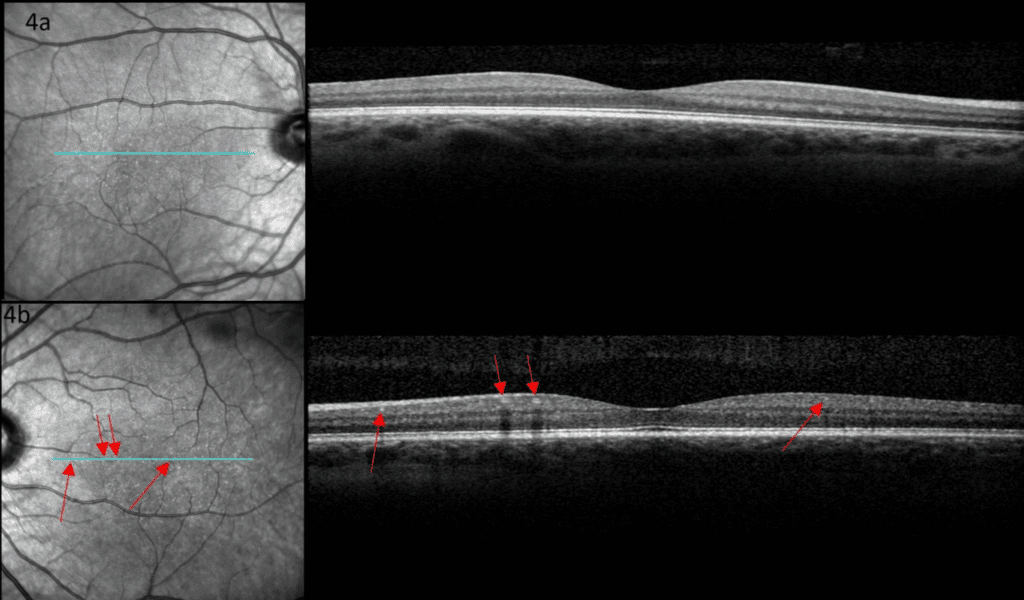
Figure 4. Optical coherence tomography of the right (a) and left (b) maculae were unremarkable. The dots were not visualized. Red arrows indicate small arterioles.
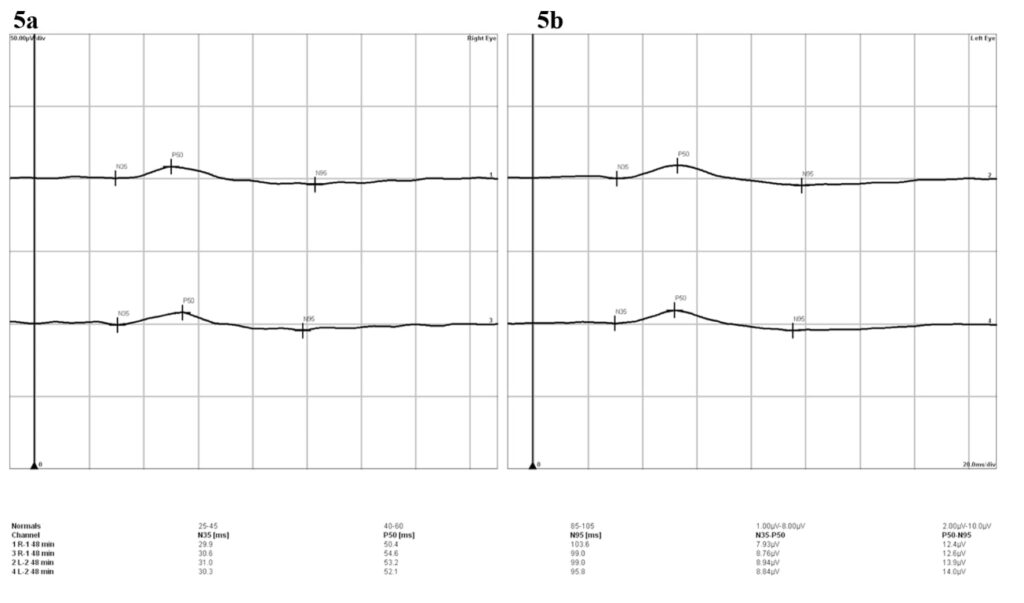
Figure 5. The results of the pattern electroretinogram revealed normal amplitudes and implicit times of P50 and N95 in both the right (a) and left (b) eyes, denoting normal macular cone and retinal ganglion cell (RGC) functions.
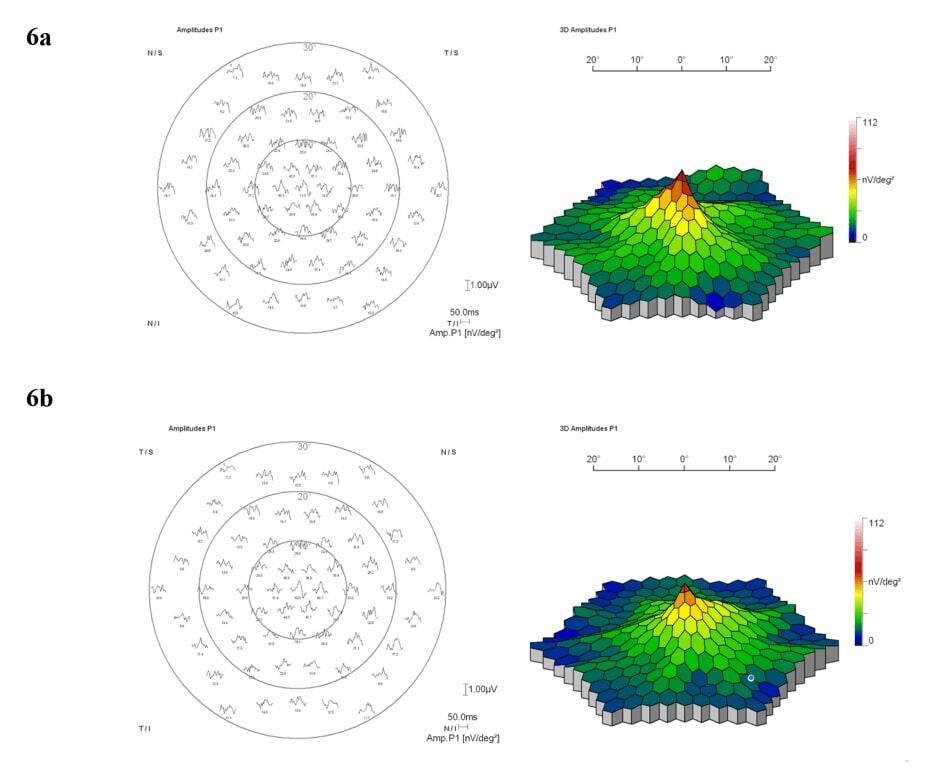
Figure 6. Trace array recordings and 3-dimensional amplitude density plots of the multifocal electroretinogram were obtained for both the right (a) and left (b) eyes. The findings indicated normal cone function in the central 30 degrees for both eyes.
Differential Diagnosis
Many macular diseases may manifest with yellow macular dots, making the differential diagnosis challenging. The potential etiologies include toxic, genetic, degenerative, idiopathic, and iatrogenic factors. The specific differential diagnoses to consider include Tamoxifen retinopathy, Talc retinopathy, Phenothiazine toxicity, Canthaxanthin maculopathy, Bietti’s Crystalline Dystrophy, Stargardt disease, pattern dystrophies, Familial Dominant Drusen, early Age-Related Macular Degeneration, and Idiopathic Macular Telangiectasia. Appendix 1 lists detailed information about signs, symptoms, and treatment of the various differential diagnoses for yellow macular dots. Given the normal visual acuity, fundoscopy exam, imaging results, and electrophysiology findings, the patient was diagnosed with Benign Yellow Dot Maculopathy.
DISCUSSION
Benign Yellow Dot Maculopathy was introduced as a new macular phenotype by Dev Borman et al. in 2017. At the time of this report, only 47 prior cases from 30 families had been detailed in seven published articles. This non-progressive condition, often discovered incidentally during examinations or identified through external referrals for evaluation, was associated with normal visual function.1–6 Females appear to be slightly more affected than males and the maculopathy could exhibit a sporadic or autosomal dominant inheritance pattern, although the causative gene remains unidentified.1 The median age of the patients was 17 years, with a range of 5-69 years.1–6
Patients typically presented with symmetric, bilateral, evenly distributed discrete yellow dots around the fovea or a more concentrated pattern in the nasal parafoveal region.1–5 However, two cases described by Mishra et al. and Balas et al. revealed the possibility of unilateral presentation.6,7
The sole consistent feature observed across all patients was hyper-autofluorescence of the dots on FAF imaging.1–6 Hyper-autofluorescence is typically caused by the accumulation of lipofuscin or other ocular fluorophores due to RPE dysfunction, the bleaching effect, or a window defect from the degeneration of overlying photoreceptors.8 For this condition, OCT was carried out in 28 cases (59%). Most patients presented normal results, but 11 individuals showed subtle irregularities in the RPE layer, interdigitation zone, or ellipsoid zone.1–6 Furthermore, 7 out of the 11 abnormal OCT scans were from the same case report by Dev Borman.1 Of these, six exhibited minimal irregularity of the ellipsoid zone, corresponding to the locations of the yellow dots.1 One abnormal OCT scan revealed mild irregularity of the RPE layer, which also matched the location of the yellow dot.1 Both unilateral cases demonstrated slight OCT changes: Mishra et al.’s case showed mild RPE irregularity, while Balas et al. suggested that the lesions were at the level of the RPE-ellipsoid zone6,7 OCT-A was conducted in seven patients, six showed no abnormalities, while one exhibited a subtle increase in choroidal vessel rarefaction at the level of the choriocapillaris.3,5,7 Intravenous fluorescein angiography (IVFA) of the fundus in six patients showed early hyper-fluorescence of the dots and stability of dots in terms of size and intensity over time.1–3 Microperimetry was performed on two patients by Murro et al. which showed normal results.3 Additionally, Murro et al. used adaptive optics scanning laser ophthalmoscope on two patients, which suggested that the yellow dots may originate from the RPE or the outer hyper-reflective retinal layers (i.e. external limiting membrane, ellipsoid zone and interdigitation zone).3 Based on current case reports, the yellow dots are likely located in the outer retinal layers; however, their exact biochemical composition remains undetermined. As non-invasive imaging technology continues to advance, it may eventually be possible to pinpoint the exact location of these lesions. Alternatively, the results could remain inconclusive until a histological examination is conducted.
Full-field electroretinography (ffERG) was conducted on 21 cases (or 46%), and all results were normal.1,3 Pattern electroretinography (pERG) was performed on 19 cases (or 41%), revealing mild or moderate macular dysfunction in only two patients.1 Multifocal electroretinography (mfERG) was performed in three cases, with only one patient exhibiting reduced peak intensity but normal implicit times.3,6
The patient presented in this case report exhibited comparable traits to those documented in the literature, including bilateral symmetric yellow dots evenly distributed perifoveally, excellent visual acuity, normal OCT, pERG, and mfERG findings, and hyper-autofluorescence of the dots on FAF and near-IR imaging. Given the benign nature and stability of this condition, intravenous fluorescein angiography and genetic testing were not conducted for this patient. This clinical team opted to observe and monitor the patient in a year and recommended a comprehensive eye examination for immediate family members of the patient.
CONCLUSION
This case of Benign Yellow Dot Maculopathy represents a unique phenotype that has limited coverage in existing literature. To date, only 48 cases have been documented, including the present case. The lack of reports on this condition might be linked to its asymptomatic and generally benign nature. Raising awareness about this emerging entity can enhance diagnostic precision, prevent misdiagnosis, and provide patients with a comforting prognosis.
REFERENCES
- Borman AD, Rachitskaya A, Suzani M, Sisk RA, Ahmed ZM, Holder GE, Cipriani V, Arno G, Webster AR, Hufnagel RB, Berrocal A, Moore AT. Benign Yellow Dot Maculopathy: A New Macular Phenotype. Ophthalmology 2017;124:1004–13.
- Moisseiev E. Benign yellow dot maculopathy. Am J Ophthalmol Case Rep 2018;10:13–5.
- Murro V, Mucciolo DP, Giorgio D, Sodi A, Passerini I, Pacini B, Finocchio L, Virgili G, Rizzo S. Multimodal imaging of benign yellow dot maculopathy. Ophthalmic Genet 2019;40:135–40.
- Kasetty VM, Desai TU, Desai UR. Benign yellow-dot maculopathy: case report and review of the literature. Can J Ophthalmol 2023;58:e191–4.
- Ninet L, David T, Gascon P. Multimodal Imaging for Benign Yellow Dot Maculopathy. Ophthalmol Retina 2022;6:307.
- Mishra A V, Pollmann AS, Choudhry N, Demmings E, Gupta RR. Unilateral benign yellow dot maculopathy. Am J Ophthalmol Case Rep 2021;22:101068.
- Balas M, Wong J, Arjmand P. Multimodal Imaging of Unilateral Benign Yellow Dot Maculopathy. J Vitreoretin Dis 2024;8:597–9.
- Yung M, Klufas MA, Sarraf D. Clinical applications of fundus autofluorescence in retinal disease. Int J Retina Vitreous 2016;2.
- Abbasi O, Tewari A. Common Medications That May Be Toxic to the Retina. Review of Ophthalmology June 2009.
- Hueber A, Rosentreter A, Severin M. Canthaxanthin retinopathy: long-term observations. Ophthalmic Res 2011;46:103–6.
- García-García GP, Martínez-Rubio M, Moya-Moya MA, Pérez-Santonja JJ, Escribano J. Current perspectives in Bietti crystalline dystrophy. Clin Ophthalmol 2019;13:1379–99.
- Tanna P, Strauss RW, Fujinami K, Michaelides M. Stargardt disease: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol 2017;101:25–30.
- van Huet RAC, Bax NM, Westeneng-Van Haaften SC, Muhamad M, Zonneveld-Vrieling MN, Hoefsloot LH, Cremers FPM, Boon CJF, Klevering BJ, Hoyng CB. Foveal sparing in Stargardt disease. Invest Ophthalmol Vis Sci 2014;55:7467–78.
- Boon CJF, van Schooneveld MJ, den Hollander AI, van Lith-Verhoeven JJC, Zonneveld-Vrieling MN, Theelen T, Cremers FPM, Hoyng CB, Klevering BJ. Mutations in the peripherin/RDS gene are an important cause of multifocal pattern dystrophy simulating STGD1/fundus flavimaculatus. Br J Ophthalmol 2007;91:1504–11.
| Factor | Description / Typical Age of Onset | Signs | Symptoms | Treatment |
|---|---|---|---|---|
| Toxic/Iatrogenic Factors | ||||
| Tamoxifen | Selective estrogen receptor modulator | Refractile yellow-white crystals scattered throughout the posterior pole primarily in the perifoveal macula, mild cystoid macular edema (CME), and retinal pigmentary changes later; may develop whorl-like, white, subepithelial corneal deposits | Asymptomatic or mild decrease in vision and dyschromatopsia | Decrease or discontinue the medication if toxicity develops. Crystalline deposits remain after drug cessation. |
| Magnesium Silicate (talc) | Vehicle for methylphenidate (Ritalin) and methadone | Refractive yellow deposits near or in arterioles in IV drug abuser. Can lead to ischemic events: optic disc neovascularization (NVD), peripheral neovascularization (NVE), vitreous hemorrhage, arteriovenous anastomosis. | Asymptomatic or severe, progressive vision loss | No effective treatment for macular ischemia |
| Phenothiazine (Thioridazine, chlorpromazine) | Antipsychotic | Mild granular pigment stippling in early stage, then progresses to salt-and-pepper pigment retinopathy. Late stage appears similar to retinitis pigmentosa | Decrease in vision, dyschromatopsia, nyctalopia, ring or paracentral visual field loss | Decrease or discontinue the medication if toxicity develops |
| Canthaxanthin | Oral tanning agents | Refractile yellow spots in a ring-shaped pattern around the fovea | Asymptomatic, or mild decrease in vision and metamorphopsia | Decrease or discontinue the medication if toxicity develops. May clear slowly over 20 years10 |
| Genetic Factors | ||||
| Bietti’s Crystalline Dystrophy CYP4V2 Autosomal recessive |
Teenage to 3rd decade East Asian ethnicities11 |
Yellow-white crystalline lipid deposits in the retina, corneal limbus, crystalline lens; RPE atrophy, sclerosis of choroidal vessel | Progressive decreased vision, nyctalopia, visual field loss, impaired color vision, ultimately legal blindness in most patients | No effective treatment |
| Late-onset foveal-sparing Stargardt disease ABCA4 Missense/hypomorphic mutation12 |
3rd to 6th decade Male = female |
Bilateral, deep, symmetric, yellow pisciform (fish-tail shaped) flecks sparing fovea. “Salt-and-pepper” pigmentary changes in periphery may develop later | Central VA at least 20/4013. Milder color and night vision changes. No photophobia | No effective treatment |
| Multifocal pattern dystrophy simulating Stargardt disease Peripherin/RDS gene Autosomal dominant |
4th to 5th decade | Irregular yellow-white flecks throughout the posterior pole and around the retinal vascular arcades, chorioretinal atrophy14 | Decreased vision, metamorphopsia, central scotoma, nyctalopia | No effective treatment |
| Dominant Drusen EFEMP1 Autosomal dominant |
2nd to 3rd decade | Bilateral, symmetric, round, yellow-white deposits throughout the posterior pole and nasal to the optic disc. The lesions coalesce forming a honeycomb appearance. May be associated with pigment clumping, RPE pigmentary disturbance, RPE detachment, chorioretinal atrophy, and CNVM | Asymptomatic unless degenerative changes occur in the macula | No effective treatment |
| Age-Related Macular Degeneration (AMD) >30 genes identified Complement cascade on chromosome 1 ARMS2/HTRA genes on chromosome 10 |
Caucasian >75 years old Family history Cigarette smoking Hyperopia Light iris color HTN HLD CVD Female > male |
Small hard drusen, larger soft drusen, geographic atrophy of the RPE, RPE clumping, and blunted foveal reflex. CNVM. | Initially asymptomatic or may have decreased vision, metamorphopsia early. Advanced atrophic or wet form may have central or pericentral scotoma | Dry AMD: AREDS 2 vitamin, green leafy vegetables, low-vision aids Wet AMD: Intravitreal anti-VEGF, focal laser photocoagulation |
| North Carolina Macular Dystrophy MCDR1 Autosomal dominant |
1st decade | Yellow drusen appear in early childhood and progress to chorioretinal atrophy, macular staphyloma or “coloboma”, and peripheral drusen | Normal central vision early unless atrophic macular “coloboma” forms; possible progression to worse than 20/200 vision in patients who develop CNVM | No effective treatment |
| Idiopathic Factors | ||||
| Type 2 Bilateral Acquired Parafoveal Telangiectasia | 5th to 6th decade Male = female |
Symmetric, bilateral, blunting or grayish discoloration of the foveal reflex, right-angle venules within 1 disc diameter of the central fovea, characteristic stellate retinal pigment epithelial hyperplasia or atrophy; leakage from telangiectatic vessels but no exudates. May eventually lead to atrophy | Mild blurring of central vision early, slowly, progressive loss of central vision over years | No treatment recommended unless CNVM develops. Extrafoveal CNVM: focal laser photocoagulation. Juxtafoveal or subfoveal CNVM: intravitreal anti-VEGF |