Ocular Tilt Reaction Associated with Infratentorial Microhemorrhages and Siderosis
ABSTRACT
BACKGROUND
Siderosis is a rare condition where hemosiderin deposition into the central nervous system leads to progressive neurologic symptoms. Hemosiderin is neurotoxic, so blood within or upon the brain in relevant regions may cause ocular tilt reaction to occur due to abnormal vestibulo-ocular input.
CASE REPORT
We report an 82-year-old man who presented with several months of various neurologic symptoms; his eye exam uncovered skew deviation and ocular tilt reaction. His exam findings ultimately led to the diagnosis of cerebellar and midbrain microhemorrhages suggestive of infratentorial siderosis.
CONCLUSION
Infratentorial microhemorrhages and siderosis can produce insidious, progressive neurologic symptoms that may mimic other neurodegenerative disorders, but must remain in the differential. A careful eye exam, history, and understanding the vestibulo-ocular system will help guide the clinician in ordering appropriate imaging.
Keywords: skew deviation, ocular tilt reaction, siderosis, infratentorial, cerebellum
INTRODUCTION
Patients with complex symptoms who have already been evaluated by several other specialties can present a diagnostic challenge. It may be easy to assume that all necessary evaluations and testing have been completed by our medical colleagues, when in fact a careful review of the case, thorough ophthalmologic clinical evaluation and additional selective diagnostic testing may hold the key to the diagnosis.
CASE REPORT
An 82-year-old patient presented to optometry service for ongoing symptoms of intermittent diplopia, “slanted” vision, nausea, dizziness and imbalance. His medical history included paroxysmal atrial fibrillation, non-ischemic cardiomyopathy, coronary artery disease status post (s/p) coronary artery bypass graft, severe aortic stenosis s/p transcatheter aortic valve replacement, hypertension, hyperlipidemia, obesity, obstructive sleep apnea and a history of deep vein thrombosis and pulmonary embolism. He was on several medications including amiodarone, amlodipine, apixaban, atorvastatin, finasteride, losartan, tadalafil and tamsulosin.
He reported that while driving six months prior, he had an acute onset of diplopia which lasted for a few minutes, so his spouse took him to a local emergency department (ED). A computed tomography (CT) of his head without (w/o) contrast had no abnormal findings, and CT Angiography (CTA) of his head and neck showed only mild atheromatous disease of the carotid arteries without significant stenosis. He had hyponatremia with sodium of 128 mmol/L, which was chronic and stable, and he was discharged the same day. One week later he followed up with his primary care physician (PCP), where he reported motion sickness. His physical exam showed some cerumen impaction in both ears, so he was given an ear irrigation kit (carbamide peroxide solution) and ondansetron 4 mg to use sparingly as needed. Three days later, he presented to the ED with two minute-long episodes of blurry vision with dizziness; his exam in the ED was unremarkable, so he was referred for an outpatient consult with ophthalmology. Five days later, he had a tele-ED visit for worsening nausea, and another three days thereafter for transient diplopia and imbalance, determined likely to be due to benign positional peripheral vertigo (BPPV), perhaps exacerbated by anticholinergic medication use. He was instructed to follow up with ophthalmology and otolaryngology (ENT). The following day, he reported to ophthalmology a vertigo-like sensation, “faint feeling,” nausea, and intermittent transient blurry vision and diplopia. Giant cell arteritis symptom screen was negative. His extraocular muscle (EOM) movements were normal, and he was noted to be ortho on cover testing, so he was referred back to his PCP. The Dix-Hallpike maneuver was negative, making BPPV less likely, and lack of positional nature made orthostasis less likely, so there was some concern for Meniere’s disease.
Over the next several months he underwent a plethora of evaluations. He was tested for COVID, which was negative. He underwent ear canal irrigation, which unfortunately resulted in an iatrogenic tympanic membrane (TM) rupture and later treatment with ciprofloxacin/dexamethasone. He separately trialed meclizine, scopolamine patches, prochlorperazine, discontinuing fluoride, altering the time of day he took his medications, and dietary changes. He consulted occupational therapy for handrails, grab-bars and a ramp for his home, and underwent vestibular rehabilitation with physical therapy. He had pre-existing sensorineural hearing loss (SNHL), and he followed up with audiology after the TM perforation, noting constant tinnitus left more than right. His pure tone audiometry indicated a 10 dB decrease at 6000 Hz on the right, and 15-35 dB decrease in thresholds 250-3000 Hz, indicating a moderate downward sloping to profound SNHL on the left. He had several ED visits for the same symptoms of intermittent blurry vision, diplopia, and nausea – now especially when looking down, bending down or moving. Another ED CT head w/o contrast and CTA head and neck showed senescent changes and narrowing of the origins of the vertebral arteries, perhaps significant on the right, but no other significant abnormalities. Ophthalmology was re-consulted, where EOMs were noted as intact, no nystagmus was present, and he was noted as orthoptic in primary, up, down, left and right gazes. He next consulted neurology, where there was consideration of symptomatic vertebral artery stenosis, so he was referred to the peripheral vascular (PV) clinic.
By the time of presentation to optometry, he had undergone quite a host of workup and intervention, none of which seemed to determine the etiology of nor improve his symptoms, which were in fact becoming more debilitating. He described imbalance, “motion sickness,” that objects all appeared “slanted,” intermittent two minute episodes of vertical diplopia, and that he was “almost instantly nauseous” anytime he tried to read or look at his phone or computer. He denied any falls or head trauma except being hit below the left ear with a golf club four decades prior without loss of consciousness. The remainder of his medical history was unchanged. He presented with an immediately noticeable 15 to 30 degree left head tilt, which his spouse had noticed, but they were unsure of when it began. He had no afferent pupillary defect and his EOM function was normal. On cover testing there was a small right hyperphoria measuring one prism diopter (PD) in primary, left, and downgazes, and in right and left tilts, 0.5 PD in upgaze and 3 PD right hypertropia in right gaze. Maddox rod testing demonstrated a very small incyclotorsion of the right eye (OD) but no detectable cyclotorsion of the left eye (OS) (Table 1). Neurologic tests heel-to-shin and finger-to-nose, tested on both sides, were normal. His eye exam was otherwise unremarkable and noncontributory. Given his eye alignment, anomalous head posture and constellation of symptoms, he was diagnosed with skew deviation and ocular tilt reaction (OTR) so magnetic resonance imaging (MRI) of his brain with and without contrast was ordered with request for special attention to brainstem and cerebellum. The patient was given 1 PD base down Fresnel prism OD on his glasses, which – unfortunately – did not improve his symptoms.
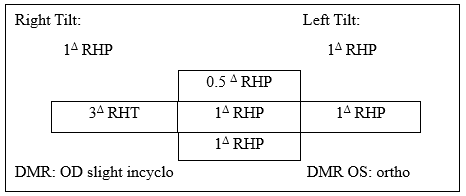
Table 1. Ocular alignment: right hyperphoria (RHP) measuring 1 prism diopter (Δ) in primary, left, and downgazes, and in right and left tilts, 0.5Δ in up gaze and 3Δ right hypertropia (RHT) in right gaze. On Maddox rod testing, he had a very small incyclotorsion of the right eye (OD), but no detectable cyclotorsion of the left eye (OS).
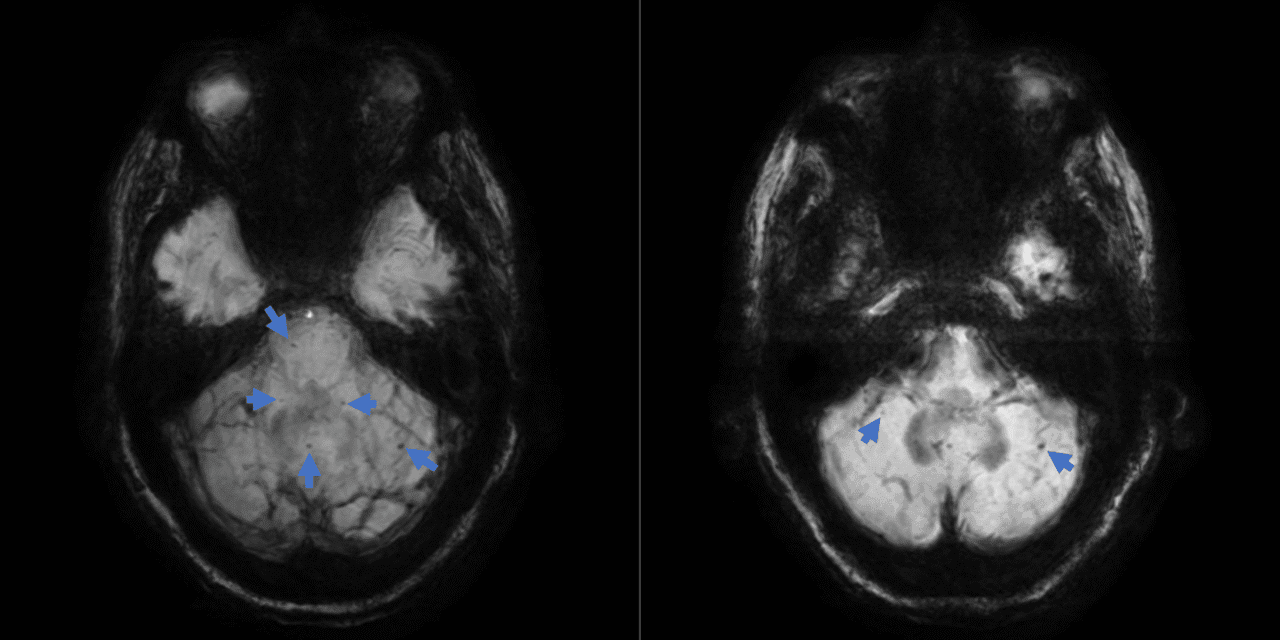
The MRI impression noted mild senescent changes and indicated no evidence of acute intracranial pathology. However, the narrative of findings included the presence of punctate foci of susceptibility artifact in the bilateral cerebellar hemispheres and cerebellar vermis, the right aspect of the midbrain, as well as a few in the supratentorial brain, consistent with chronic hemosiderin deposition (Figure 1). With this information and correlation to clinical exam, microbleeding and infratentorial siderosis became the presumed etiology for his symptoms.
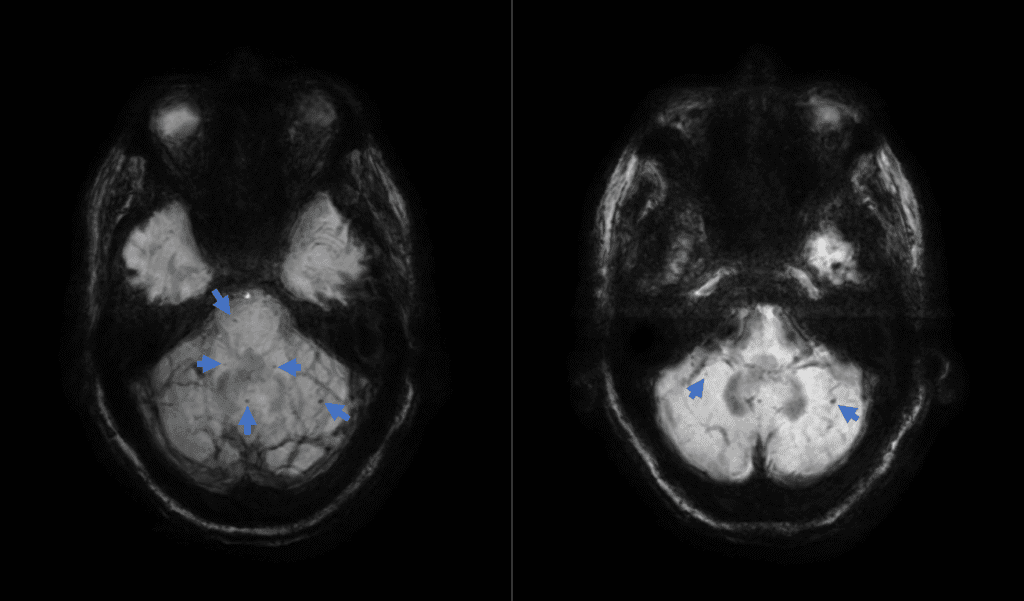
Figure 1: Axial MRI susceptibility-weighted imaging with minimum intensity projection images showing scattered foci of hemosiderin deposition (arrows) in the cerebellar vermis, cerebellar hemispheres and right aspect of the midbrain.
This case highlights the importance of understanding the patient’s symptoms, a careful eye exam, understanding localization and which ancillary tests would be most revealing, and a high level of suspicion—even when at face value a test is read as normal. Interdisciplinary communication is also paramount. After reviewing the MRI and its interpretation, discussion with radiology helped to corroborate exam findings and symptoms with the MRI findings. With this data, the patient’s integrated healthcare team worked to manage symptoms and consider the best course for his overall health. Anticoagulation increases the risk for hemorrhages—and therefore siderosis—compared to aspirin, so risk versus benefit of anticoagulation versus aspirin monotherapy was considered by the patient and his team. Ultimately, the plan was to consider a Watchman device with post-surgical dual antiplatelet therapy (aspirin and clopidogrel) followed by aspirin indefinitely while discontinuing apixaban to lessen the risk for recurrent intracranial bleeding. Audiology was also alerted given the worsening of his SNHL. His case and management are ongoing.
DISCUSSION
Skew deviation and Ocular Tilt Reaction
To understand skew deviation and OTR, it is helpful to understand supranuclear oculomotor pathways. The vestibulo-ocular system connects pathways from the inner ear to areas in the midbrain that initiate eye movements. The goal of the vestibulo-ocular system is to keep the fovea of each eye fixated on a target despite head or body movement. This system begins in the otoliths in the semicircular canals on each side, travels through the vestibular nerves to the vestibular nuclei in the brainstem, then to the oculomotor nuclei to control conjugate eye movements. For example, with a normal vestibulo-ocular reflex (VOR), as one’s head turns right, the eyes move left to maintain foveation of a target. If there is damage to the inner ear or vestibular nerve on one side, the ipsilateral damaged VOR will cause the eyes will drift towards the affected side, followed by a quick restorative reset of the eyes to orbital center, thus manifesting as a jerk nystagmus with the fast phase opposite the affected side.1
Different oculomotor nuclei are recruited to employ the VOR for upward eye movement. Near the oculomotor nucleus in the midbrain are the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) and the interstitial nucleus of Cajal (INC). The riMLF initiates vertical gaze, whereas the INC is responsible for keeping the eyes in vertical gaze against elastic orbital forces that would otherwise draw the eyes back towards center.1 For example, normally, when one’s head tilts to the right, the VOR begins with activation within the ipsilateral inner ear, the signal is sent to the vestibular nucleus and through the MLF to connect to the INC; this directs the right eye to move up and intort, and the left eye to move down and extort, keeping the eyes parallel to the horizon (Figure 2).1

Figure 2: Example of normal VOR: as the head tilts right, the OD moves up and incyclotorts, the OS moves down and excyclotorts (e.g. both eyes rotate opposite the tilt).
Interruption – or imbalance – of pre-oculomotor nuclear vestibular input to the INC leads to ocular tilt reaction (OTR). OTR is classically known as a triad of skew deviation, head tilt towards the side of the hypodeviated eye, and bilateral ocular torsion in the same direction as the tilt (Figure 3). The torsional component does not usually produce symptoms but may cause vertical (e.g. y-axis or subjective visual vertical) to appear tilted towards the hypodeviated eye.1,3 This subjective tilting is fairly pathognomonic for OTR.1 The patient in this case described his field as appearing “slanted.” The vertical ocular misalignment component—known as skew deviation—usually leads to vertical diplopia.1 The magnitude of the skew misalignment can vary substantially, but is typically relatively small, generally 5 PD or less; when 3 PD or less, patients tend to report blurry vision rather than frank diplopia.2
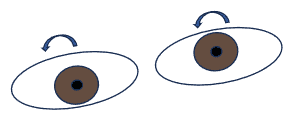
Figure 3: Example of OTR due to left-side MLF lesion: as the head tilts right, the OD moves down and excyclotorts, the OS moves up and incyclotorts (e.g. both eyes rotate opposite the affected side).
Skew deviation alone is a vertical ocular misalignment that does not localize to any cyclovertical extraocular muscle and occurs in association with neurological signs or symptoms.3 Skew deviation is measured as a comitant hypertropia in nearly two-thirds of cases but it can also be incomitant. Skew deviation alone seldom has a torsional component, but when present, it is usually incyclotorsion of the hyperdeviated eye and excyclotorsion of the hypodeviated eye—this is unlike a more common trochlear nerve palsy which shows excyclotorsion of the hyperdeviated eye.1-3 An alternating skew deviation, where OD is hyperdeviated in right gaze and OS is hyperdeviated in left gaze, is present in around one-third of skew deviations and is usually accompanied by saccadic pursuits and nystagmus. Intermittent or paroxysmal skew deviation is also possible, but rare.1,2 Skew deviation can be siloed but is typically seen in conjunction with OTR.
Diagnosing skew deviation involves measuring vertical misalignment, generally by cover test, in all directions of gaze, including right and left tilt. Measuring rotational/torsional status of the eye is best achieved by the double Maddox rod test (DMR). In DMR, a red Maddox rod is placed over one eye and a white Maddox rod over the other, with both rods’ parallel lines (cylinders) oriented vertically. While looking at a single white light, the patient will see two horizontal lines, red and white, respectively when there is no cyclotorsion of either eye, and the line from the hyperdeviated eye will appear lower. When an eye is excyclotorted, the line will appear perceptually slanted downwards towards their nose and appear slanted downwards towards their ipsilateral ear if the eye is incyclotorted. The patient is asked to rotate the rods until the two lines appear parallel to each other, aligned with the horizon. The result demonstrates both the magnitude and direction of the cyclotorsion. The relative rotation away from 90 degrees of the Maddox rods indicates the degrees of cyclotorsion of the eye(s). The patient will rotate the top of the rods’ cylinders towards their ipsilateral ear for an excyclotorted eye, and towards their nose for an incyclotorted eye (Figure 4). Torsion can also be assessed based on fundus imaging if needed.4 Signs or symptoms suggestive of vestibular, diencephalic, brainstem or cerebellar dysfunction are present in skew deviation, though they may be subtle. Therefore, clinical context is important in making a skew diagnosis.
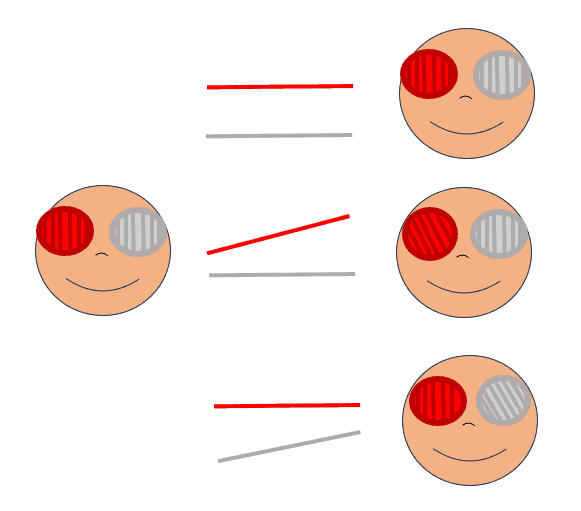
Left Panel: DMR set up with red and white Maddox rods positioned with cylinders oriented vertically over right and left eyes, respectively. Middle Panel: Examples of patient’s view with DMR set up. Top: Left eye hyper, no cyclotorsion. Middle: Left eye hyper, right eye excyclotorted. Bottom: Left eye hyper, left eye incyclotorted. Right panel: Doctor’s view of patient’s response on DMR (e.g. when patient subjectively makes the lines appear parallel). Top: Left eye hyper, no cyclotorsion. Middle: Left eye hyper, right eye excyclotorted. Bottom: Left eye hyper, left eye incyclotorted.
The Upright-Supine Test is an effective differentiator between skew and other causes of vertical misalignment. This test involves using a target about arm’s length away, measuring vertical deviation while in an upright position, then re-measuring with the head supine. This could be with either the entire body supine, or the head only (e.g. chin up/head back). A decrease in the vertical deviation by at least 50% when supine compared to upright is considered a positive test and is compatible with skew deviation. Wong et al found this test to be 100% specific and 80% sensitive in differentiating skew from other causes of vertical strabismus, such as trochlear palsies, restrictive causes, myasthenia gravis and others.4
Skew deviation or OTR can be localized to peripheral or central insults in addition to occurring after peripheral vestibular damage (e.g. inner ear or vestibular nerve); any unilateral or asymmetric diencephalic, cerebellar or brainstem lesion leading to vestibular tone imbalance can cause a central skew deviation or OTR. This includes the medulla, pons, cerebellum, MLF, midbrain, thalamus and vestibular cortex.3 Bilateral INC lesions cause seesaw nystagmus. 1 As an example of a central skew deviation, if the patient had had a cerebrovascular accident (CVA) affecting the left MLF, the INC would then receive asymmetrical input, leading to OTR where OD would rotate contralateral to the affected side (e.g. excyclotort) and hypodeviate, and OS would also rotate contralateral to the affected side (e.g. incyclotort) and hyperdeviate, and the head would tilt to the right (Figure 3).1
Around half of skew deviations are due to thalamus, brainstem or cerebellar ischemic CVA; these insults may be very small and may not be evident even on high-resolution MRI imaging, and the location of the lesion does not dictate comitance/incomitance or alternation of the skew deviation.2 Other culprits include hemorrhage, mass lesions or demyelination.1 Neurologic signs accompanying skew deviation include nystagmus or ataxia in around half of cases, gaze paresis, saccadic pursuits, or intranuclear ophthalmoplegia, among many others which may localize to any level of the brainstem or cerebellum.2,5 Over half of patients have accompanying non-visual symptoms including dizziness or imbalance (around one-quarter of patients), headache, weakness, nausea, etc. Around 11% of patients may have only skew deviation with no other neurologic signs or detectable neuroimaging abnormalities.2
In the largest retrospective series on skew deviation of 157 cases by Walter et al, skew deviation spontaneously resolved in around 42% of patients, especially if due to demyelination, but was least likely to resolve if due to brainstem tumors, aneurysms of the posterior circulation or cerebellar disorders unrelated to a mass. More debilitating than the skew deviation were the accompanying neurologic signs like ataxia that did not resolve in over 40% of patients. Ataxia is more likely to resolve if due to demyelination and least likely to resolve in cerebellar disorders or traumatic brain injury. When resolution does occur, it can be delayed by a year or more. Nearly two-thirds of patients with skew may have alleviation of diplopia with vertical prism glasses; interestingly, neither the cause of the skew deviation nor the magnitude or comitance were predictive of who would experience symptom relief.2 Other therapeutic options include strabismus surgery or patching.
Superficial Siderosis
Superficial siderosis (SS) is a rare condition whose diagnosis is made radiologically, showing hemosiderin deposition in the subpial layers of the brain, spinal cord and central nerves.6 Hemosiderin, a blood component involved in iron delivery, is neurotoxic, leading to progressive neuronal and glial atrophy—especially in the cerebellum, which is rich in Purkinje cells and microglia.7,8 The most affected regions in SS are the cerebellum and brainstem, which are both exposed to high levels of cerebrospinal fluid (CSF) where hemoglobin levels increase in the context of chronic subarachnoid bleeding. The cerebellum’s large surface area may render it more susceptible to the deposits of hemosiderin.9 The cerebellum houses 50-75% of the neurons in human brains and their cells have very high metabolic demand, making them very sensitive to metabolic imbalance.8 Cranial nerves are also susceptible to SS, especially the vestibulocochlear nerves (i.e. cranial nerve, CN VIII) with their long glial segment and high CSF exposure through the pontine cistern.9
Most patients with SS present with overt manifestations including SNHL from CN VIII damage, and ataxia in nearly 90%, along with pyramidal signs and other cerebellar deficits. Siderosis is a rare and slowly progressive condition, so it may not initially be considered in the differential diagnosis for someone presenting with these symptoms, or the clinical findings may be attributed to other neurodegenerative diseases.6,10 CT imaging is not sensitive in detecting SS, whereas it is obvious on MRI where the hemosiderin demonstrates marginal hypointensity on T2-weighted imaging and hypointensity of the brain surface is seen on T2 gradient recalled echo and susceptibility-weighted imaging.8,11
While SS may be related to explicit causes of subarachnoid hemorrhaging such as trauma, it is more often attributable to various sources of insidious microbleeds such as dural tears and nearly 35% of cases are idiopathic.6,7,12 While stopping the source of bleeding, surgically or otherwise, is an obvious goal to halt further injury, existing damage and symptoms are generally irreversible, and the condition tends to slowly progress regardless.7 Off-label administration of deferiprone, an iron chelator which is lipid soluble and can permeate the blood-brain barrier, has been employed, but outcomes both clinically and radiologically are mixed and adverse effects may be serious, including agranulocytosis.8,12
Any intracranial bleeding will leave evidence of hemosiderin deposits. This patient did not have hemosiderin superficially on the brain’s surface as in SS but rather had evidence of multiple small hemorrhages leaving hemosiderin deposition within the midbrain and cerebellum. Both the microbleeds (essentially tiny hemorrhagic strokes) themselves and hemosiderin-associated neurotoxicity (siderosis) within these infratentorial regions likely contributed to this patient’s symptomatology. His skew deviation and OTR may have been directly from the right midbrain bleed, which is in the vicinity of the oculomotor nuclear complex, or could have been related to defunct vestibulo-ocular input from the cerebellar microhemorrhages and siderosis.
CONCLUSION
Disruptions to the delicate vestibulo-ocular system can lead to visual complaints, and the source of that disruption may also result in other neurologic signs and symptoms. This patient’s infratentorial microbleeds also triggered a progression of neurologic symptoms including skew deviation with OTR, which steered appropriate imaging to lead to the correct diagnosis. Siderosis can produce insidious, progressive neurologic symptoms that may mimic other neurodegenerative disorders, but must remain in the differential.
REFERENCES
- Trobe J. Neuro-Ophthalmology at your Fingertips [Internet]. Ann Arbor (MI): University of Michigan, Department of Ophthalmology and Visual Sciences. Available from: https://fingertips.neuro-ophthalmology.med.umich.edu/. Accessed 11 Mar 2025.
- Walter E, Trobe J. The Clinical and Imaging Profile of Skew Deviation: A Study of 157 Cases. J Neuro-Ophthalmol 2021 Mar;41(1):69-76.
- Hernowo A, Eggenberger E. Skew deviation. Clinical updates for ophthalmologists. Curr Opinion Ophthalmol 2014;25(6):485-7.
- Wong A MF, Colpa L, Chandrakumar M. Ability of an Upright-Supine Test to Differentiate Skew Deviation From Other Vertical Strabismus Causes. Arch Ophthalmol. 2011;129(12):1570-5.
- Keane JR. Ocular skew deviation. Analysis of 100 cases. Arch Neurol 1975 Mar;32(3):185-90.
- Vernooij MW, Ikram MA, Hofman A, Krestin GP, et al. Superficial siderosis in the general population. Neurology. 2009 Jul 21;73(3):202-5.
- Hoskin J, Kaneko K, Munakomi S, Fife T. Superficial Siderosis. StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK603744/. Accessed 13 Mar 2025.
- Ismail FY, Mitoma H, Fatemi A. Handbook of Clinical Neurology. Chapter 8. Metablic ataxias. 2018. 155;117-27.
- Lee S-Y. Lee D-H, Bae YJ, S J-J, et al. Bilateral Vestibulopathy in Superficial Siderosis. Front Neurol. 2018 June 6;9:422.
- Castelli ML, Husband A. Superficial siderosis of the central nervous system: An underestimated cause of hearing loss. J Laryngeal Otol. 1997;111:60-2.
- Offenbacher H, Fazekas F, Schmidt R, Kapellar P, et al. Superficial siderosis of the central nervous system: MRI findings and clinical significance. Neuroradiology. 1996;38:551-6.
- Martin AF, Shanmugarajah P, Hoggard N, Hadjivassiliou M. Treatment Response of Deferiprone in Infratentorial Superficial Siderosis: A Systematic Review. Cerebellum. 2021 Jan 6;20(3):454-61.