
Pseudoxanthoma Elasticum and Angioid Streaks
ABSTRACT
INTRODUCTION
Angioid streaks are associated with a few rare systemic disorders. Pseudoxanthoma elasticum (PXE), the most common systemic disorder associated with angioid streaks, is a rare autosomal recessive disorder caused by a mutation in the ABCC6 gene. Angioid streaks increase the risk of choroidal neovascularization, subretinal hemorrhage and subsequent vision loss. Fundus autofluorescence (FAF) imaging highlights changes that are commonly associated with PXE.
CASE REPORT
A 59-year-old female patient presented with bilateral angioid streaks, choroidal neovascularization, and yellowish skin papules on the lateral and posterior side of her neck which led to a PXE diagnosis.
CONCLUSION
PXE is a critical multisystem disease that often presents with readily visible dermatologic manifestations and clinically typical fundus exam findings; optometrists are in a key position to diagnose this condition and refer these patients for interdisciplinary consults, possibly saving their lives.
Keywords: pseudoxanthoma elasticum, angioid streak, Bruch’s membrane, choroidal neovascularization, subretinal hemorrhage
INTRODUCTION
Angioid streaks can be seen in systemic disorders such as Paget’s disease of bone, sickle cell disease, β-thalassemia, and Ehlers-Danlos syndrome. However, the most common systemic disorder associated with angioid streaks is pseudoxanthoma elasticum (PXE).1–6 PXE is a rare, autosomal recessive, multisystem disease with a prevalence of 1:50,000 affecting approximately 150,000 people worldwide.4,7 Being autosomal recessive, a sibling of an affected individual has a 25% chance of manifesting PXE themselves. It affects females more than males at a ratio of 2:1, and its clinical manifestations only become evident in the second or third decade of life.4 PXE is caused by a mutation in the ABCC6 gene, which is expressed in the liver and the kidneys. It is believed that the inactivity of ABCC6 in the liver causes low amounts of anti-mineralization factor to circulate, leading to uninhibited accumulation of calcium-phosphate complex in the connective tissues, as well as degeneration and fragmentation of elastic fibers.4 These elastic fiber abnormalities involve the skin, eyes and the cardiovascular system.
CASE REPORT
A 59-year-old Hispanic female patient presented to establish care, complaining of chronic, gradually progressive blurry vision. She reported not being able to see out of her left eye for the last five or more years. She had been followed at a different institution for about two years for a “genetic disease,” and had received ten intravitreal aflibercept injections in her left eye (OS) in the past, and one in her right eye (OD) three months ago; she was also status post epiretinal membrane peel in both eyes (OU).
The patient denied ocular trauma, hearing difficulties, bone disorders or blood dyscrasias. She had one brother and one sister with longstanding reduced vision in each eye of unknown etiology. Her medical history was remarkable for hypertension, type 2 diabetes mellitus, hyperlipidemia and hyperthyroidism. Her medications included nifedipine, empagliflozin, atorvastatin, methimazole and atenolol. The patient denied the use of alcohol or tobacco and reported no known medication or environmental allergies. Her best-corrected visual acuity was 20/30 OD, 20/300 OS; she had a small left exotropia by Hirschberg test. There was no afferent pupillary defect.
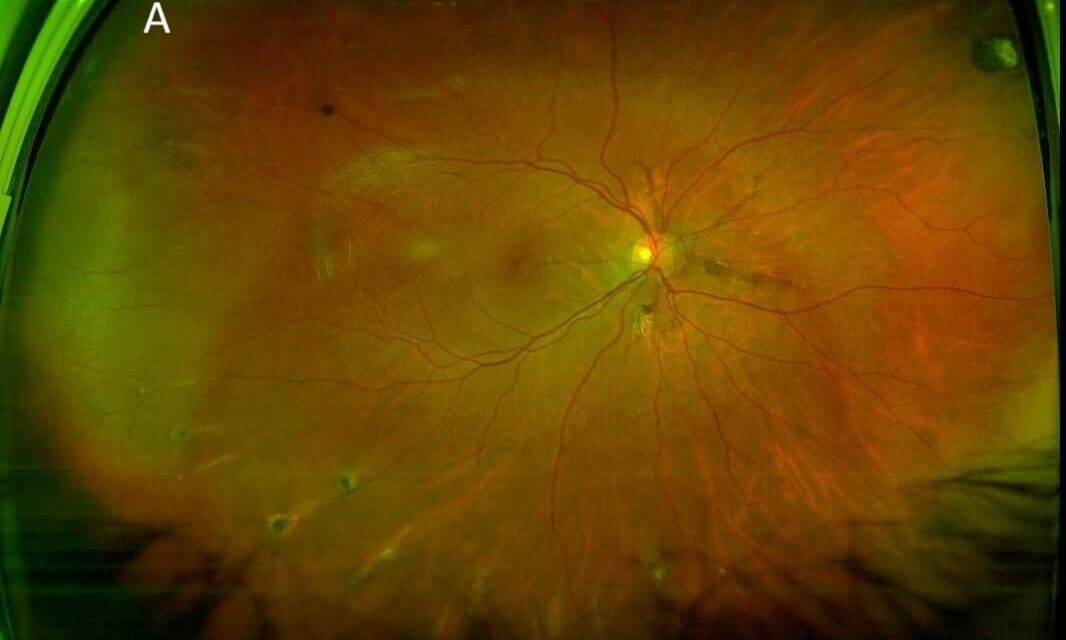
The dilated fundus exam was remarkable for reddish-brown curvilinear breaks in Bruch’s membrane radiating from the optic nerve head OU, consistent with angioid streaks (Figure 1), subretinal hemorrhage (SRH) and exudation in the macula OD and SRH with overlying fibrovascular scarring in the macula OS. There were also multiple comet-shaped atrophic chorioretinal lesions OU highlighted on fundus autofluorescence (FAF) imaging (Figure 2). Retinal blood vessels appeared attenuated but otherwise were unremarkable. Macular optical coherence tomography (OCT) showed type 2 choroidal neovascularization (CNV) (Figure 3), which worsened in OD in the interval between the initial visit and when she was able to receive another intravitreal bevacizumab injection about one month later at her previously scheduled retina appointment (Figure 4). Skin-colored papules on the side and back of the patient’s neck were noted (Figure 5). With this constellation of clinical findings, a diagnosis of PXE was established.
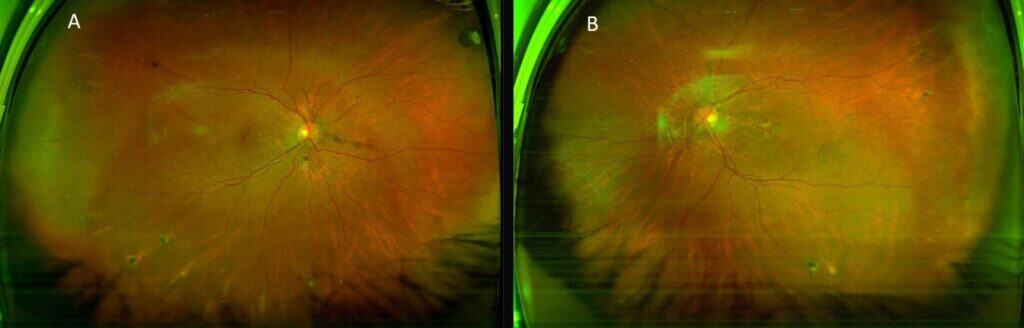
FIGURE 1: Optos red/green imaging of OD (A) and OS (B) showing curvilinear reddish-brown angioid streaks radiating from the optic nerves, multiple comet lesions in the periphery, and submacular hemorrhage and fibrosis (left eye).
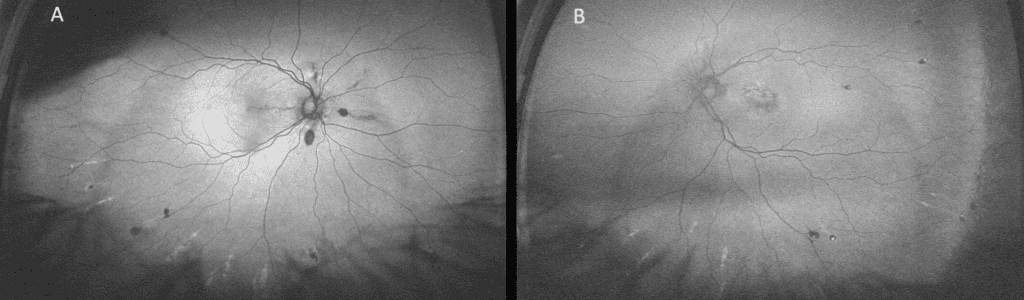
FIGURE 2. Optos FAF imaging of OD (A) and OS (B) showing hypo-AF of angioid streaks radiating from the optic nerves and comet lesions in the periphery with hypo-AF center and hyper-AF surround.
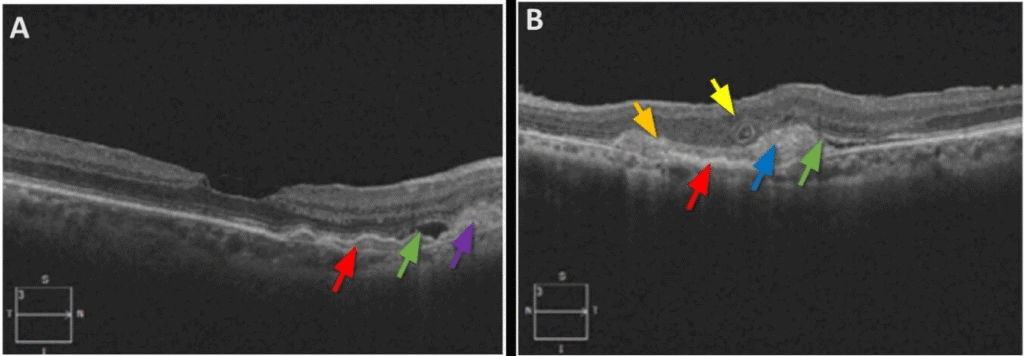
FIGURE 3. Cross-sectional OCT of the macula OD (A) and OS (B) shows consequences of type 2 CNV: (A) areas of sub-RPE (red arrow) and subretinal (purple arrow) heterogenous, moderately reflective hemorrhage and subretinal fluid (green arrow); (B) sub-RPE (red arrow) and subretinal (blue arrow) heterogenous debris with medium and high reflectivity (SRH with some subretinal fibrosis); break in Bruch’s membrane (orange arrow), and outer retinal tubulation (yellow arrow).
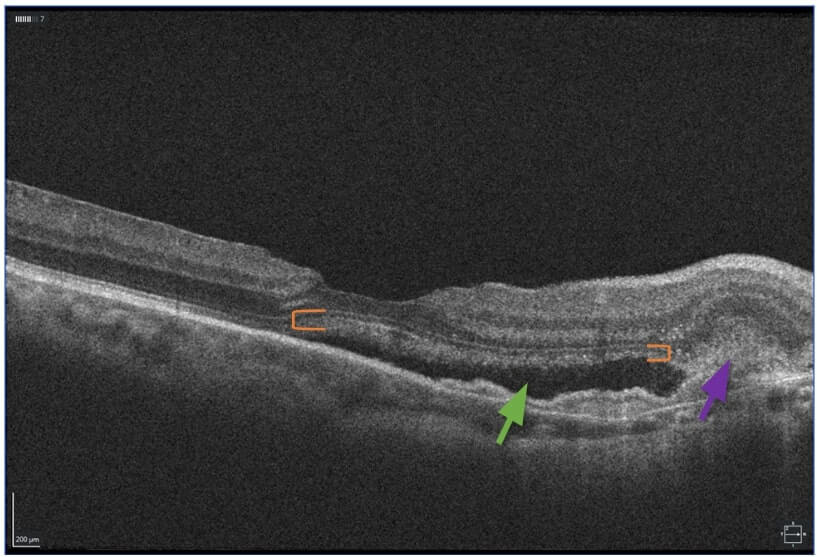
FIGURE 4. OCT OD showing an increase in subretinal fluid (green arrow) and sub-RPE- and SRH (purple arrow) in the interval until she was able to receive another intravitreal bevacizumab injection about 1 month later. Shaggy photoreceptors (orange brackets), due to unphagocytized outer segments, signifying chronicity.
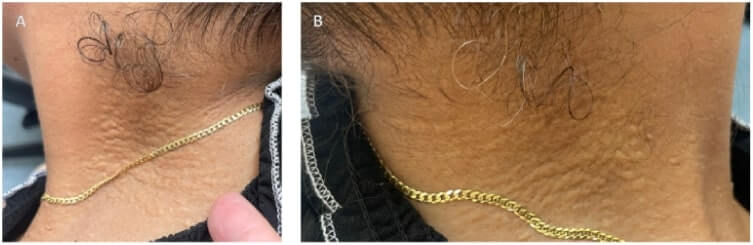
FIGURE 5. External Photograph of right side (A) and left side (B) of patient’s neck showing “plucked chicken” skin-colored papules that are consistent with dermatologic manifestation of PXE.
DISCUSSION
The first signs of PXE involve the skin, with the appearance of small yellowish asymptomatic papules on the lateral and posterior areas of the neck, and flexural areas such as the periumbilical region, axillae, antecubital and popliteal fossae, and the inguinal area.4 The small papules can coalesce into a larger plaque with time and often have a “plucked chicken” skin appearance. Dermatologic manifestations of PXE may pose cosmetic problems, but the ocular and cardiovascular complications pose significant morbidity, and at times mortality, risk for patients.
In the eyes, PXE causes thickening and fragmentation of Bruch’s membrane from calcific deposits in the elastic fibers. These cracks in Bruch’s membrane appear as reddish-brown lines radiating from or close to the optic nerve head in the posterior pole, and are referred to as angioid streaks.6 Even minor blunt injury to eyes with angioid streaks can lead to SRH. Other common complications associated with angioid streaks are CNV, SRH, exudation and fibrovascular scar formation, which, when involving the macular region, causes significant morbidity due to loss of vision or visual function. Additional fundoscopic features of PXE include peripheral atrophic chorioretinal lesions in the shape of comets with their tails pointed towards the optic nerve head. On fundus autofluorescence imaging, these comet lesions have a hypoautofluorescent (hypo-AF) center with hyperautofluorescent (hyper-AF) surround.4 PXE also manifests with “peau d’orange” pigmentary changes in the temporal mid-peripheral retina. 6 There is also an increased prevalence of optic disc drusen in patients with PXE.4,6 This may be due to an increased propensity for calcification in both PXE and optic disc drusen.
Patients with CNV secondary to PXE require treatment with anti-vascular endothelial growth factor (anti-VEGF) intravitreal injections at an earlier age when compared to patients with exudative age-related macular degeneration (AMD); they also manifest CNV in the fellow eye more quickly compared to patients with exudative AMD.8 If best-corrected visual acuity (BCVA) is already reduced due to CNV in PXE before anti-VEGF is initiated, poorer visual outcomes result.8 So, it is recommended that CNV in patients with PXE be monitored closely by a retinal specialist and receive intervention as quickly as possible with longer-acting anti-VEGF agents.
In addition to PXE, other associated conditions must be considered in a patient with angioid streaks. About 6% of patients with sickle cell hemoglobinopathies manifest angioid streaks.6,9 Additionally, sickle cell disease may lead to segmentation of blood in conjunctival vessels, retinal thromboemboli, and complications of retinal ischemia such as “salmon patch” hemorrhages or peripheral “sea fan” neovascularization.
Angioid streaks related to high myopia, often called “lacquer cracks,” frequently occur in the presence of posterior staphyloma, peripapillary atrophy of the retinal pigmented epithelium (RPE), tilted discs, myopic macular degeneration, areas of retinal thinning, schisis, or widespread chorioretinal degeneration that are often associated with high myopia.6,10
Paget’s disease of bone can be associated with angioid streaks; it has a predilection for people of European descent and affects males more than females. Patients with Paget’s disease of bone often complain of hip, thigh, leg, or lower back pain and may have hearing impairment related to involvement of the skull base. Approximately 40-50% of patients with Paget’s disease of bone report a positive family history, but only 10% acquire the disease sporadically due to a mutation in SQSTM1.11
Ehlers-Danlos syndrome (EDS) is an extremely rare genetically inherited disorder of the connective tissue that affects multiple organs. People with EDS have lax skin that is easily bruised, hypermobility and proprioception difficulties. Additional ocular manifestations of EDS include myopia, keratoconus, zonular laxity or dehiscence, or scleral thinning, among others, related to collagen abnormalities in these tissues.[1]
Choroidal ruptures may appear similar to angioid streaks; however, traumatic choroidal ruptures are typically concentric around the optic nerve head, rather than radial.6
In an effort to understand the underlying mechanism of the PXE-associated mutation in the ABCC6 gene, Ran and colleagues evaluated the biochemical and biophysical properties of the N-terminal nucleotide-binding domain 1 (NBD1) of the human ABCC6 gene and found a correlation between alterations in NBD properties and the ABCC6 gene’s activity.1 More specifically, mutations involving D777N and D778N were shown to be critical in the folding process and for the structural stability of the ABCC6 gene (Figure 6). Inactive and unstable ABCC6 has been linked to mineralization and fragmentation of elastic fibers in the connective tissue of patients with PXE.1
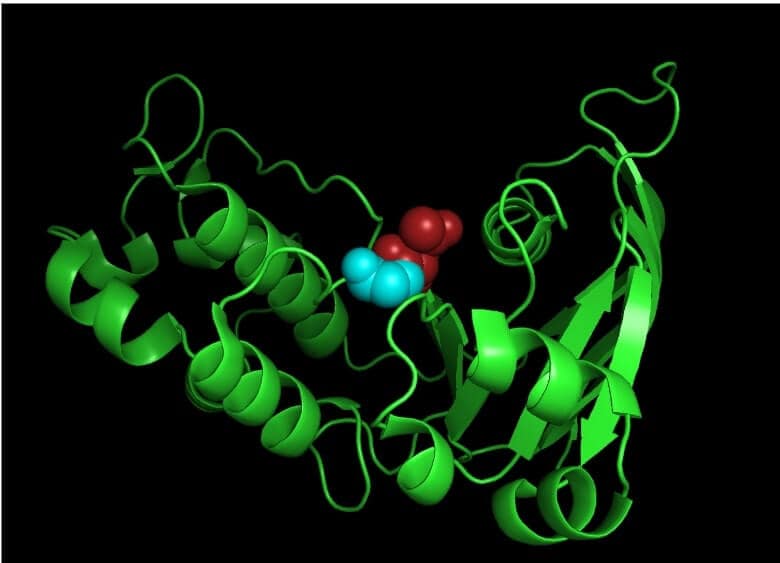
FIGURE 6. Pictorial representation highlighting D777N (red spheres) and D778N (cyan spheres) mutation in NBD1 of human ABCC6 gene. This representation was created using PyMOL software.
Because PXE affects the elastic fibers of blood vessels, it may cause major or life-threatening complications, such as gastrointestinal (GI) hemorrhages or calcific atherosclerosis of coronary or cerebral arteries; complications of this vascular calcification may include microvascular occlusion, vessel dissection, local thromboembolic occlusion within affected arteries, or downstream emboli – all of which may lead to morbidity or mortality including myocardial infarction (MI) or cerebrovascular accidents (CVA). Approximately 13% of patients with PXE develop hemorrhages or blood clots in their stomach and upper intestine.12 An esophagogastroduodenoscopy (EGD) or angiography is performed to diagnose GI hemorrhages; treatment includes embolization or coiling.12 The association between PXE and cardiac complications appears to be low, but patients may develop a cardiopathy later in life. 13 Patients often undergo a physical examination, electrocardiogram (EKG), cardiac magnetic resonance imaging (MRI), transthoracic echocardiography (TTE), treadmill testing, or perfusion myocardial scintigraphy (SPECT) by a cardiologist.13 Even though there are case reports of PXE patients reporting upper and lower limb claudication, the occurrence of peripheral artery disease (PAD) in PXE patients is low. Additionally, the success rate of interventional procedures such as endovascular or bypass surgery is also low overall.14 This could be because of the low prevalence of PXE in general. Patients diagnosed with or suspected of having PXE should be referred to gastroenterologists, cardiologists or peripheral vascular specialists for further evaluation.
CONCLUSION
Pseudoxanthoma elasticum (PXE) is an autosomal recessive condition caused by a mutation in the ABCC6 gene; it affects females at a 2:1 ratio. PXE leads to deposition of calcium-phosphate complexes in the elastic fibers of connective tissue, causing fragmentation, affecting tissues in the skin, eyes and blood vessels. Skin-colored or yellowish papules on the sides or the back of the neck, retinal comet lesions, and bilateral angioid streaks (often with associated CNV or SRH) are characteristic features of PXE. Full-time protective eyewear is recommended for PXE patients, given that even minor ocular trauma can lead to SRH in the presence of angioid streaks. Referrals to evaluate other organ system involvement are recommended.
References
- Ran Y, Zheng A, Thibodeau PH. Structural analysis reveals pathomechanisms associated with pseudoxanthoma elasticum–causing mutations in the ABCC6 transporter. J Biol Chem. 2018;293(41):15855-15866. doi:10.1074/jbc.RA118.004806
- Schachat AP, ed. Ryan’s Retina. Sixth edition. Elsevier; 2018.
- Wubben TJ, Besirli CG, Johnson MW, Zacks DN. Retinal Neuroprotection: Overcoming the Translational Roadblocks. Am J Ophthalmol. 2018;192:xv-xxii. doi:10.1016/j.ajo.2018.04.012
- Germain DP. Pseudoxanthoma elasticum. Orphanet J Rare Dis. 2017;12(1):85. doi:10.1186/s13023-017-0639-8
- Whelan L, Dockery A, Wynne N, et al. Findings from a Genotyping Study of over 1000 People with Inherited Retinal Disorders in Ireland. Genes. 2020;11(1):105. doi:10.3390/genes11010105
- MCCANNEL CA. 2022-2023 BASIC AND CLINICAL SCIENCE COURSE, SECTION 12 Retina and Vitreous Print. AMER ACADEMY OF OPHTHALMO; 2022.
- Salmon JF, Kanski JJ. Kanski’s Clinical Ophthalmology: A Systematic Approach. Ninth edition. Elsevier; 2020.
- Raming K, Pfau M, Herrmann P, Holz FG, Pfau K. Anti-VEGF Treatment for Secondary Neovascularization in Pseudoxanthoma Elasticum – Age of Onset, Treatment Frequency, and Visual Outcome. Am J Ophthalmol. 2024;265:127-136. doi:10.1016/j.ajo.2024.03.026
- McLEOD DS, Merges C, Fukushima A, Goldberg MF, Lutty GA. Histopathologic Features of Neovascularization in Sickle Cell Retinopathy. Am J Ophthalmol. 1997;124(4):455-472. doi:10.1016/S0002-9394(14)70862-1
- Zhang W, Zhang Y, Xu J, Dan H, Li X, Song Z. A physical sign of pathological myopia: myopic scleral pit. BMC Ophthalmol. 2023;23(1):114. doi:10.1186/s12886-023-02847-y
- Ralston SH. Paget’s Disease of Bone. N Engl J Med. 2013;368(7):644-650. doi:10.1056/NEJMcp1204713
- Qian SS, Kesar V, Shah F, Park D, Sorrentino D. Pseudoxanthoma Elasticum. ACG Case Rep J. 2020;7(6):e00410. doi:10.14309/crj.0000000000000410
- Prunier F, Terrien G, Le Corre Y, et al. Pseudoxanthoma Elasticum: Cardiac Findings in Patients and Abcc6-Deficient Mouse Model. Lionetti V, ed. PLoS ONE. 2013;8(7):e68700. doi:10.1371/journal.pone.0068700
- Verwer MC, Hazenberg CEVB, Spiering W, De Borst GJ. Peripheral Interventions in Patients with Pseudoxanthoma Elasticum (PXE). Eur J Vasc Endovasc Surg. 2023;65(1):142-148. doi:10.1016/j.ejvs.2022.08.009












