A pilot crossover study comparing yoked and split prisms for collision judgment asymmetry after stroke

ABSTRACT
Significance
Age is a major risk factor for large-vessel stroke, which results in left visuospatial neglect in as many as 25% of cases. Visuospatial problems are a major barrier for returning to independent mobility.
Purpose
Damage to right brain spatial attention networks causes mobility impairment, such as veering, imbalance, collisions, and falls that often persist chronically. A possible cause of these impairments is a rightward deviation in egocentric coordinates, referred to as visual midline shift syndrome or representational left visuospatial neglect (LVSN). A rightward deviation should result in a leftward veering trajectory as well as collisions with detected obstacles due to spatial misjudgment. Base left yoked prisms have been used anecdotally and in small open-label studies to correct the veering and collisions; however, yoked prism shifts the view of the body and the scene together. If the body is visually referenced to make decisions about collision risk during mobility, yoked prisms will have no effect. To address this, this study proposes a split-prism design that takes advantage of the dominant role of online visual feedback during ambulation, shifting the view of the body left and the visual scene to the right. To test the relative impact of prism designs, a virtual reality collision judgment paradigm was used, which has been previously shown to consistently characterize rightward errors in subjects with LVSN on a realistic task relevant to everyday mobility.
Methods
Six subjects with LVSN with rightward asymmetry in their collision judgments (Mean 35.2 cm) performed collision judgments in virtual reality with yoked prism (either 4∆, 7∆, or 20∆ base left) and split prism (10∆ BL/12∆BR). The view around the lenses was occluded, and subjects were seated with their arms resting on the chinrest table, so they were visible in their inferior visual field. Data were analyzed using mixed-effects models to determine if significant changes in the symmetry of collision judgments occurred.
Results
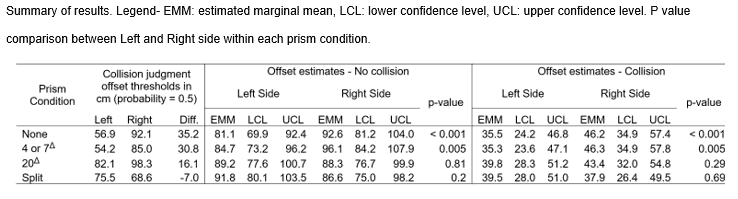
Mean baseline asymmetry was 35.2 cm rightward. The 4Δ or 7Δ yoked prisms did not have an effect and split prism had the greatest effect towards normalizing or even reversing the asymmetry, resulting in a 7 cm leftward asymmetry. A 20Δ yoked-prism also reduced asymmetry, to 16.1 cm rightward, perhaps due to minification (distortion) near the base, which was substantial in this flat prism.
Conclusion
This pilot study supports further study of split prisms for veering during mobility and imbalance in LVSN after stroke.
Background
Stroke is the leading cause of serious long-term disability in the United States, with an estimated 4.7 million of the 7 million survivors currently disabled.1,2 Large vessel strokes account for 22-56% of all presenting strokes, but cause 42-81% of the post-stroke disability (2 to 3.8 million Americans).3 Left Visuospatial Neglect (LVSN) is a common stroke-related disorder of visuospatial cognition. It occurs in about half of right brain large vessel strokes,4-6 as well as 30% of intracerebral hemorrhage from falls and other forms of severe traumatic brain injury.5,7 Classic signs of LVSN include failure to don clothing on the left side of the body, failure to read from the beginning of the sentence, failure to eat food from the left side of the plate, or “forgetting” the left arm or leg. In testing, patients typically err to the right on line bisection testing, fail to cancel symbols on the left side of a page, and show left-side incompletion when copying a shape.8 Inpatients with LVSN were found to have a 6.5-fold increased fall risk during inpatient rehabilitation, and LVSN was an independent predictor of long-term rehabilitation outcome.9,10
In terms of mobility, LVSN is associated with a reduced ability to maintain a correct trajectory (veering), postural abnormalities with deviated center of pressure when seated or standing, and frequent collisions. These issues represent major barriers for return to independent mobility in the long-term.11,12 In some cases, veering and postural abnormalities may be due to a rightward deviated perception of body midline (egocenter), referred to as representational LVSN or visual midline shift syndrome (VMSS). A rightward angular error in body representation would be predicted to result in leftward veering and left-side collisions (Fig. 1B and 1C); however, prior studies found mixed results, which might be explained by interactions between motor and visual perceptual impairments.11, 13-26 Virtual reality (VR) offers a method to isolate visual impairments from motor impairments to target errors in visuospatial processing and evaluate the effects of targeted visual treatment.27
In a prior study by the author, 26 participants with a history of stroke and 21 normal controls performed collision judgments in a virtual reality shopping mall.27 The LVSN group exhibited a median 27 cm (4.4°) rightward asymmetry in their collision judgment thresholds (smaller threshold on left and trended towards larger threshold on the right). Eight out of the 10 LVSN cases showed >10 cm (~2°) of asymmetry, likely to be clinically relevant. In a mobility course study, a subset of this sample showed a positive relationship between walking speed and asymmetry (i.e. those with more asymmetry walked more slowly), suggesting real-world relevance to the abnormal collision judgment measurements.28 These results supported the existence of a deviation in the internal representation of body size or body position specific to patients with a history of right brain stroke, which is called VMSS in optometric literature and representational LVSN in neurology and neuropsychology literature. More importantly, in this study, the deviated judgments occurred consistently and persistently over several months of data collection during a task relevant to everyday mobility: collision judgment. The very existence of this condition has been a topic of controversy in the fields of optometry and ophthalmology, and this well-controlled yet ecologically relevant study was important to confirm that egocentric midline shift exists, is not rare, and it chronically impairs collision judgments of right-brain stroke survivors. This was the first study to document VMSS using the most rigorous psychophysical methodology, the method of constant stimuli, to produce psychophysical collision thresholds.29 Studies prior to this had only used method of adjustment, asking subjects to verbally report when a visual stimulus was straight ahead, which is prone to directionality effects, habituation, and prediction behaviors.30-33
This present study built upon this prior work by determining if the collision judgment asymmetry in patients with LVSN could be improved using prismatic lenses to counteract the error.
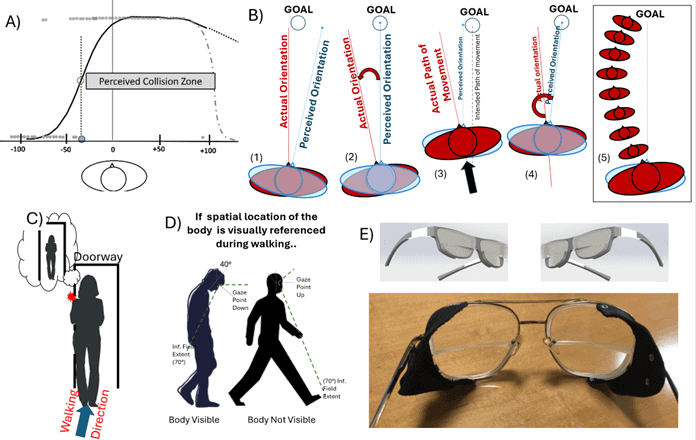
Figure 1. (A) Collision judgment plot from data in a virtual shopping mall illustrating a LVSN subject with large rightward collision judgment asymmetry. A model of rightward angular deviation in representational egocenter is proposed to explain this finding. (B) Model showing how rightward angular deviation in the truncal egocenter would result in leftward veering. (1) The patient has a rightward error in their perceived truncal midline. (2) This causes them to err in the opposite direction when aligning the perceived truncal vector to the locomotory goal. (3) The patient moves on the incorrect vector until they detect the error, whereupon (4) they make a correction again aligning the incorrectly perceived midline. (5) This process repeats resulting in a veering path to the left. (C) Angular deviation in egocenter may cause spatial misjudgments which cause left-side collisions, i.e. patient perceives their body position as illustrated in bubble, that they will pass safely, and then collide with the obstacle. The patient sees the obstacle yet they still collide with it. (D) Illustration of the visual referencing hypothesis. This study proposes that the inferior visual field is frequently used to monitor body position, particularly when careful navigation is needed in tight quarters. It is possible that for periods of time when the head is up the body is out of the inferior field of view as shown, so some combination of visual and proprioceptive monitoring is likely during periods of walking. (E) Schematic and photo of split prisms used in this study, with 10Δ (5.7°) base left (rightward deviating) upper lens, and 12Δ (6.7°) base right (leftward deviating) lower lenses. These take advantage of visual referencing to specifically target LVSN related errors in egocentric space.
Prismatic Correction of Veering and the Visual Referencing Hypothesis: Prescription base left yoked prism glasses worn full time, which shift the entire visual field to the right, have been used for veering and postural leaning in LVSN for decades by optometrists working in neuro-rehabilitation, with supporting evidence from anecdotal reports and a few small open label studies.30-35 There are multiple potentially valid criticisms surrounding the use of these lenses:
- Studies of yoked prism in normal and stroke populations have found that adaptation to full-field yoked prisms occurs in a matter of minutes so that any beneficial effect on veering and collisions due to spatial misjudgments may rapidly decay.36-37
- Yoked prisms shift the view of the body and visual scene together, so if visual referencing of body position is the primary mechanism utilized to guide ambulation and avoid collisions, the lenses are unlikely to alter collision judgments (Figure 2).38
This study refers to this as the visual referencing hypothesis. This hypothesis requires that the primary mechanism for monitoring body position during mobility is via real-time or “online” visual monitoring. The term “online” as used here refers to constant visual monitoring (having no relation to the common internet-related use of the term). Another possibility is that body position is primarily monitored by non-visual proprioceptive information or “online” via an internal model of body position. A primarily online visual mechanism for the control of action was described and defended by Zhao and Warren in 2015.39 In that report, the substantial computational demands required to construct, maintain, and rapidly access an offline internal model of body position were dismissed as being illogical when compared to the use of the much more efficient and accurate online visual feedback.39 The authors did not deny the existence of some internal reference model for situations where vision is not available, as would be required in dark environments, when looking up, or persistently by individuals with blindness, but that in the normally-sighted these serve a minor role.
This leading theoretical framework concerning the visual control of locomotion was utilized to try and design a better lens for symptoms of representational LVSN. This approach utilizes a split prism spectacle design. The upper lens is essentially identical to yoked prisms used clinically; however, split prism spectacles contain a lower prism lens with the base in the opposite direction to visually shift the view of the body to the left (Figures 1E, 2, and 3). In theory, this causes the wearer to think their body is further to the left than it truly is, and in the case of rightward deviated perception, will counteract the rightward error. This can solve the issue that traditional yoked prisms might not change collision judgments due to shifting view of body and scene in the same direction, as well as the expected issue with rapid decay of effects due to adaptation.36 Unlike yoked prisms, adaptation to split prisms requires weeks to months of full-time wear.40,41 An additional benefit of this study is that it will provide new information to either support or oppose the online visual action model.39
The visual referencing hypothesis for the treatment of representational LVSN was tested in this prospective, single-blind randomized crossover study by comparing changes in rightward collision judgment asymmetry with split and yoked prisms of a few different strengths. The hypothesis of this study was that there would be no effect of yoked prism of any strength and that split prism would improve collision judgment asymmetry by an amount equivalent to the sum of the upper and lower prism values.
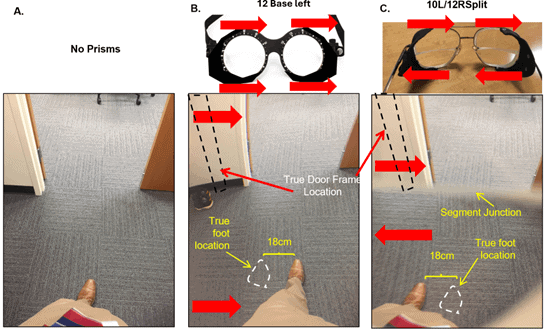
Figure 2. Photos of body view without prism and through actual yoked and split prism. A) No prism view. Subject aligned themselves to pass through the center of the doorway. B) View through yoked prism, 12∆ base left. Notice that the relative positions of leg and door frame do not change compared to (A). A moderate amount of distortion is evident as the curving of the left door frame and carpet squares. C) Photo taken through split prisms. The segment was positioned higher than it might be for actual use, to better visualize the effect. Notice the left leg now appears much further to the left, and the doorway is shifted right. This is expected to have an additive (upper + lower prism displacement) effect on collision judgments and doorway navigation, counteracting the pathological leftward veering in LVSN.
Methods
The study protocol was approved by the institutional review board and the study was conducted in accordance with the tenets of the Declaration of Helsinki. Subjects provided informed consent after receiving an explanation of study procedures and risks. Data was collected and the study completed in 2016, prior to the implementation of the FDAAA Final Rule. A portion of the data has been published previously as an American Academy of Optometry Conference Abstract.38
Subjects
Subjects had a history of first-ever unilateral large vessel cerebrovascular accident (CVA) greater than three months prior, a diagnosis of LVSN in their clinical records, or a positive Schenkenberg Line Bisection Test42 (LBT) or Bells43 test during study intake. Diagnosis of LVSN is known to be challenging in milder cases, requiring multiple tests. As such, if a diagnosis of LVSN was documented in the medical record in the prior six months, the subject was eligible even if LBT and Bells tests were negative, which was the case for two subjects (Table 1). Subjects were also required to show a rightward asymmetry in their collision judgments, measured in virtual reality (see later sections for details). This finding is itself diagnostic, as a prior study found significant deviation only in subjects with right brain stroke with diagnosis or history of LVSN, and not in a control group of stroke cases without that history.25 Visual acuity had to be at least 20/40 binocularly, uncorrected (as split prisms were non-prescription) with normal visual fields on the right side. All but one subject had some degree of left sided homonymous field defects; however, this was not required to participate. Seven subjects with LVSN were screened and six met the criteria and volunteered for enrollment (Table 1). All subjects had vision problems from their CVA and reported mobility problems as their presenting complaint. An 11-item subset of the independent mobility questionnaire44 was available for four subjects who had completed it for another study, providing a semi-quantitative characterization of mobility problems (Table 1, last column). The questionnaire required the subject to rate their level of difficulty on 11 different mobility tasks, such as stepping off a curb or moving around in crowded situations, on a scale of 1-5, with 5 representing the most difficulty, giving a maximum possible mobility impairment score of 55.
Experimental Setup and Design
This was a prospective single-blind active controlled crossover study design with order of lens conditions balanced. Subjects were blinded to the experimental condition (split prisms) versus the active controls (yoked prisms). Data collectors could not be blinded to condition as they had to properly fit and adjust the prism glasses, requiring an understanding of the design and optics.
Virtual Mall Walking Simulation Set-up and Methods
Subjects were seated with their arms resting on the chinrest table (Figure 2), so their arms were visible in their inferior visual field. Subjects sat, rather than standing or walking, to avoid confounding variables such as motor impairment and balance when performing the collision task. A chin-head rest was attached to a dark tabletop 77 cm from a rear-projection screen (170 x 125 cm) displaying a shopping mall corridor virtual model that simulated walking at 1.5 m/s (Figure 3A). Head position was stabilized via a head-chinrest, limiting postural shifts, and keeping the head aligned with the virtual path’s center. A prior published study reported use of this experimental setup for a normal vision and control group, demonstrating no collision judgment asymmetry, and confirming that rightward deviation in a LVSN group was not due to a physical offset of the setup.27 Subjects were free to scan their eyes. Instructions to subjects were to “imagine that they were walking down the mall hallway and to report if they would make contact with an obstacle—a realistically-sized human figure which appeared in the hallway—if they were to continue along the walking path without adjusting their body or changing direction to avoid the obstacle.” During each trial, an obstacle appeared at a virtual distance of 5 m and disappeared at 3.5 m whereupon the walking stopped and a verbal response of “collision,” “no collision,” or “nothing seen” was required. There were 80 such trials, 40 per side, with the side and offset of the obstacle from the walking path randomly varying from 0.2 to 1.2 m. Practice was conducted prior to data collection until the study technician was satisfied that the subject understood the task and was giving reasonable responses (at least 20 trials). Eight additional trials had no obstacles shown to monitor reliability. One subject, S3, showed poor reliability (multiple false positives), but no other subjects demonstrated such issues.
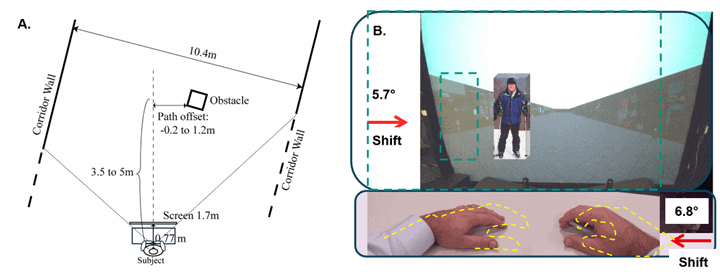
Figure 3. A) Schematic of the experimental setup showing virtual environment distances. B) Photo-illustration from the subject’s point of view on the split prism trials. The glasses and patient’s anterior/posterior head tilt (pitch) were adjusted until the junction between upper and lower prism was positioned so the hands and table would be seen through the lower base right prism (image shifted left), and the screen would be seen through the upper base left prism such that the image of the screen was shifted right. Dashed teal outline of the virtual mall and obstacle represents the veridical (true, non-shifted) position. The yellow outline of the hands represents their veridical position. In the full lens base left yoked prism trials, not illustrated, both the hands and the screen would be shifted right and so no change in collision judgments was predicted.
Prism Conditions
1) No Prism: Wearing habitual spectacles if needed;
2) Low Powered: 4Δ or 7Δ base left yoked prism;
3) High Powered: 20Δ base left yoked prism; and
4) Split: 10Δ base left upper/12Δ base right lower.
Yoked prisms consisted of rotatable prism goggles of 4Δ corrected curve, 7Δ corrected curve, or 20Δ flat custom fitted with side occluders (Figure 2B). It had been suggested in professional conversation with clinical prescribers of yoked prism that a lower power 4Δ corrected curve prism may have a larger effect than higher power 7Δ prisms due to the lens working on an unconscious level (ambient processing). The rationale may be that the patient does not notice the effect of the lower prism power, and so cognitive mechanisms of strategic adaptation are not engaged. The analysis found no difference between 4Δ and 7Δ, so data for these two lens powers were pooled and analyzed together. Split prisms were produced by cutting lenses in half and then bonding them together with optical-grade epoxy, with the upper lens being plano with 10Δ Base Left (BL) and the lower lens being plano with 12Δ Base Right (BR) (Figure 2C). Patients did not wear refractive correction for any of the conditions, as the inclusion criterion of 20/40 uncorrected vision or better. This was necessary in this small pilot study, as financial resources limited the ability to produce custom prescription split prisms for each subject. The split prism values shifted the upper field 5.7° creating a 35 cm shift of the obstacle in virtual space at the disappearance point (3.5 m) (Figure 3B). The lower 12∆ prism segment shifted the lower field by 6.8°, creating a 5 cm of shift of the view of the hands, assuming a 45 cm eye-to-hands distance (Figure 3B). As such, a change in the collision judgment asymmetry of 40 cm was predicted. Since typical yoked prisms shift the view of the scene and the body in the same direction, it was expected that there would be no change in collision judgments (Figure 2B). Subjects were first tested without any prisms, and then with yoked prism (either 4∆, 7∆, or 20∆) with the view around the lens being occluded and 10∆ BL/12∆BR split prism. The order was balanced. The view outside the split prism was also occluded (Figure 2C). Five-minute breaks were provided between testing sessions, which allowed for any effects of the prior prism trial to dissipate.
Statistical Methods
Binary responses were recorded for “collision response = 1” or “no collision response = 0.” Failure to detect was coded as “no collision” since behavior between “no collision” and “failure to detect” was the same. Prior research has shown that excluding these detection failure trials did not affect the collision judgment asymmetry or interpretation of results.27
The offset of the obstacle from the walking path was a continuous variable (measured in cm). There were two additional factors relevant to the analysis: prism condition (four levels, as described above) and obstacle side (Left or Right).
Since there were repeated measurements within subjects, mixed-effects regression models were used, with subject levels modeled as random intercepts. Two different kinds of regression analyses were performed:
- Mixed-effects logistic regression: to determine collision judgment offset thresholds, separate models were fit within each prism condition. The dependent variable was collision response, with the obstacle side and the offset value as fixed-effect predictors. The interaction between offset and obstacle side was included in the model. Collision offset thresholds for each side were obtained from the resulting fit by computing the offset corresponding to the collision probability = 0.5. The difference between the Left and Right side thresholds represented the asymmetry in collision response.
- Linear mixed-effects regression: the offset was also analyzed by a linear mixed effects model, with side, prism condition, and collision response as fixed factors. The interaction between offset and obstacle side was again included in the model. Estimated marginal means and 95% confidence interval limits were obtained from the fitted model and compared between Left and Right side within each prism condition, stratified by collision response. P-values < 0.05 were considered as statistically significant. Given the relatively small sample size, marginally significant results p < 0.1 were also mentioned. All statistical analysis was done using R 4.0.4.
Results
There were a total of 2176 trials across 6 subjects. Data was missing for 1 subject each in the 20Δ and split prism conditions, and for 2 subjects in 4Δ or 7Δ condition, due to testing fatigue. Of the 2176 trials, 950 (44%) were perceived as collisions and 1,179 as no collision. A small portion of the overall trials, 47(2%), were no response (failure to detect) and were assigned to the no collision class for the purpose of analysis. There was no effect of prism order on collision judgments, confirming the efficacy of order balancing.
Results of the analyses are in Table 2, Figure 4, and Figure 5, and key findings are summarized here. As expected, without prisms, there was a large rightward asymmetry of 35.2 cm related to the LVSN, with large differences in the left- and right-sided collision thresholds (p < 0.001). There was no appreciable change with low-powered yoked prisms, with the asymmetry remaining high at 30.8 cm, p = 0.005. The asymmetry was alleviated with the 20Δ yoked prisms (p = 0.81) and split prisms (p=0.20); non-significant values represent no difference in collision threshold on left and right sides. The split prisms provided substantially more change in asymmetry than the 20Δ yoked prisms, to the point that the asymmetry was reversed (-7 cm), representing a change of 42.2 cm, very close to the 40 cm predicted by the additive prism shift 10Δ upper and 12Δ lower (for calculations, see prism conditions in the Methods section). The yoked 20Δ produced (35.2cm-16.1cm=19.1cm) of change, (tan Θ=0.191/3.5= 3.1°) of change which is only (3.1°/11.4° *100= 27.4%) of the prism power.
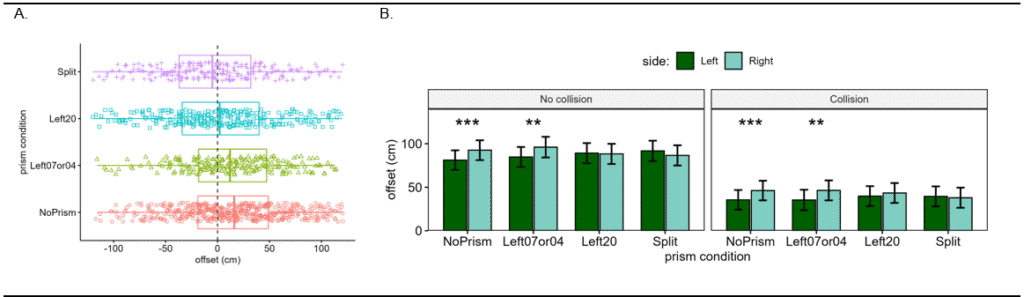
Figure 4. A) Distribution of collision judgment offset distances for detected collisions across the 4 conditions. Note that the median collision response is shifted right for the no prism and 4 Δ or 7Δ prism conditions. Split and 20Δ have median near 0 (symmetric). B) Bar plots of the estimated marginal mean offset, which was significantly larger towards the Right side for no prism and 4 or 7Δ for both collision and no collision responses, representing rightward asymmetry. For 20Δ and split prisms, the asymmetry was eliminated, i.e. there was no significant difference between the offset for the Left and Right sides for both collision and no collision responses.
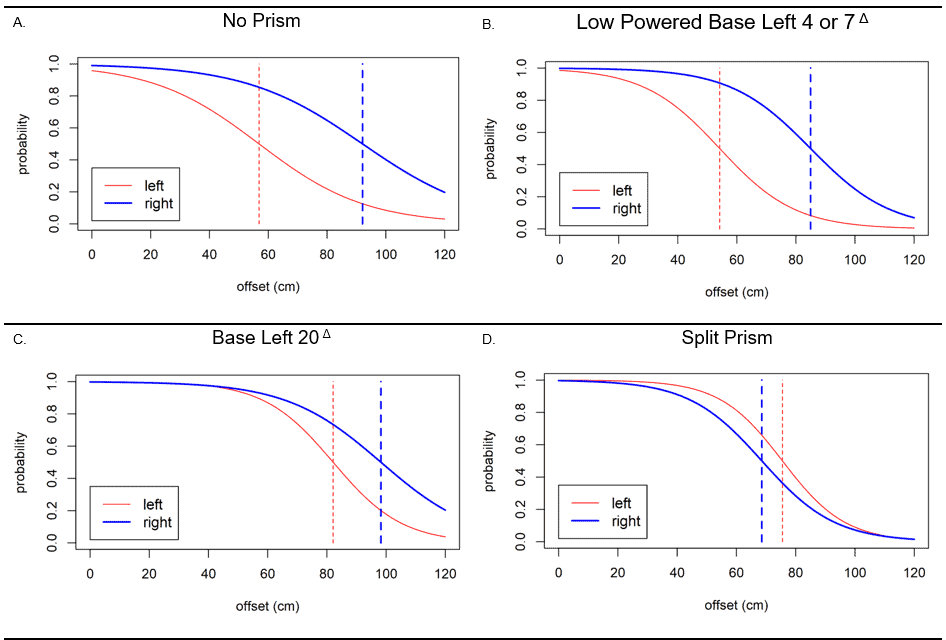
Figure 5. Collision judgement thresholds generated for the entire group of subjects for left (red) and right (blue). A) No prism condition showing asymmetry between the two sides (separation between the two dashed lines). B) Yoked 4Δ and 7Δ prisms with similar asymmetry. C) Yoked 20 Δ prism with reduced asymmetry. D) Split prism with reversed asymmetry (left (red) collision threshold is now larger than the right side (blue)).
Discussion
The primary question this research addressed was whether rightward collision judgment asymmetry caused by representational LVSN, sometimes called visual midline shift syndrome, could be improved with split prism glasses that contain a base left yoked prism in the upper part and yoked base right lower prism in the lower part. Results support the primary hypothesis that split prisms are effective to eliminate the asymmetry, and the effect was highly consistent with the apical angle of the prisms. As hypothesized, yoked base left prisms 4-7∆, which shifted the body and the scene together, had no measurable effect. Unexpectedly, the yoked base left 20∆ had an effect, although only 27.4% of the prism power, and substantially less effect than the split prisms. The fact that there was an effect of 20∆ at all may be explained by distortion in these high-powered flat prisms. The distortion is characterized by minification at the base and magnification at the apex, which is evident in the photo in Figure 2B taken through a 12∆ base left prism. Notice the rightward curved appearance of the door frame and carpet squares. This distortion effect could conceivably make the left side pedestrians appear a little closer to the walking path than they actually are. The rightward curved appearance of the walking path caused by the distortion could also counteract leftward veering. That said, the effect of the 20∆ lenses is of little clinical relevance because they are too thick and heavy to be used as prescription lenses. Still, the concept of purposefully increasing distortion in the upper split prism lens through the use of a flat or reversed base curve could be investigated.
In addition to harnessing distortion effects, there may be other ways to improve split prisms. Customization of the lens power based on the amount of asymmetry could allow some patients to have a lower power device. Under-correction could also be considered. It may be that a lower power split prism providing only partial correction would be enough to resolve most related mobility issues, while offering a lighter weight and cosmetically more acceptable device. Higher powers of the lower prism and Fresnel designs may be tolerable if patients are not fixating directly through the lens (referencing using their inferior peripheral field). In general, the positioning of the split will likely be quite important for success. If it is too high, it will interfere with central vision. If there is a gap between the frame and the face, the intended effect of the lower prism is unlikely to be obtained. To effectively implement the lower prism, a wraparound frame design (Figure 6A) or dress frame designs with a high pantoscopic tilt and low back vertex distance would be needed. Alternatively, custom 3-D printed frames could be designed to precisely place the lower prism and lens junction while minimizing view outside the lens, using craniofacial scans as has been done successfully for vision rehabilitation spectacle devices previously.45 Additionally, the upper prisms could be provided as a clip on (Figure 6B). This allows for correction only when needed for walking. Prisms add extra weight and cause some blurriness due to aberrations. The ability to remove the prism for non-mobility tasks may be appreciated by patients.
It has also been considered that prescription yoked prisms may be acting as a split prism, since the view of the body under the frame is not shifted (Figure 6C). This may represent an important difference from prior laboratory yoked prism studies, including this one, where the view around the lens was always blocked.36,37 If prescription yoked prisms are acting as a split prism, it could explain the beneficial effects reported anecdotally and in small clinical studies.30-35 If further studies support the comparative efficacy of split prisms, neuro-optometrists may wish to carefully consider the impact of frame selection and vertex distance in order to maximize the treatment effect.
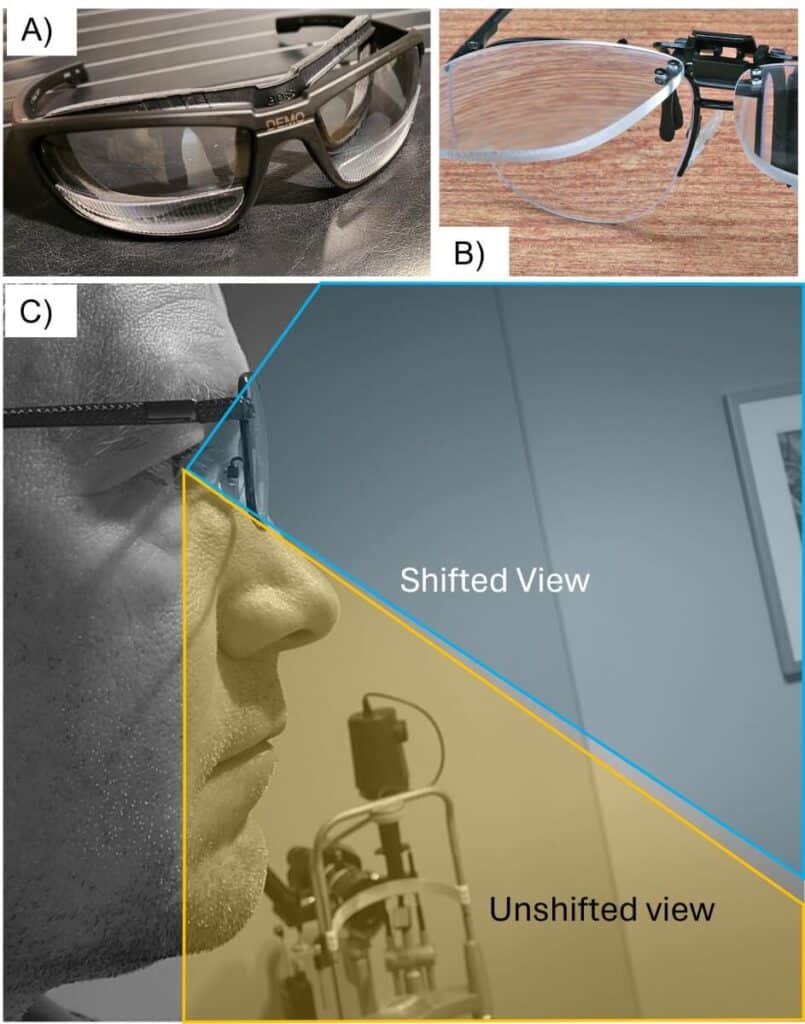
Figure 6: Methods to implement split prisms that might be utilized clinically. A) The Fresnel on the wrap frame only modifies egocentric space. B) A clip-on could be utilized to apply prism that only modifies extra-personal space. C) Yoked prisms as commonly prescribed by optometrists may also work as a split prism, explaining numerous prior anecdotal reports of success.
Many patients with LVSN also have left homonymous field defects (HFDs). In this study sample, five of the six subjects had HFDs. Split prisms and yoked prisms do not affect the visual field substantially, and results here should not be confused with HFD sector prism treatment approaches to expand the visual field and improve detection of obstacles, such as the Peli lens or Gottlieb lens.46 The impact of the HFD in this collision judgment scenario was minimal with only 2% detection failures, so the issue of collision judgment asymmetry is not related to detection failures from HFDs; it was desirable and necessary for subjects to detect the obstacle to be able to make a collision judgment. A different scenario would be needed to evaluate the impact of HFDs, as it is overtaking types of pedestrian interactions that have the highest risk for collision for patients with HFDs.47 It is possible if not likely that the LVSN subjects in this study also have some problems with obstacle detection in their daily lives due to the HFD. Peli lenses could be incorporated into the split prism glasses to address both problems. There is likely to be some combined effect on veering which requires additional study, since peripheral prisms have been found to affect collision judgments.48
The findings support the online model as the primary mechanism for visual control of action, consistent with Warren and Zhao’s 2015 work.39 If vision of the body was not being utilized to make collision judgments, there should not have been such a large effect of the split prism. This suggests that any intervention which can enhance the left side view of the body should improve symptoms of LVSN.
Limitations
There are some important limitations of this pilot study to acknowledge. While the data available was extensive due to the repeated measures, the highest psychophysical methodology was used, and significant results were found, the sample size was only 6. This sample size is appropriate at this pilot stage and supports further research. The sample in this study was derived from a clinically relevant population and was clearly defined; however, the study needs to be repeated in a larger clinical population to obtain further evidence before considering clinical translation of this approach.
Another limitation is that the study was underpowered to detect the small effect that may be present with low-power yoked prisms. The likelihood of finding an effect, even in a larger sample, is low as the effect seen in 20∆ was likely from distortion, and these lower power prisms do not have a meaningful amount of distortion. That said, this is an important clinical question that needs to be definitively answered. Professional conversations suggest that most neuro-optometrists who are prescribing yoked prisms for visual midline shift are prescribing these lower powers. The effect they are observing anecdotally may be explained by patients having a view of their body outside the prism lens, acting like a split prism. Future studies could better address this important question using the data from this present study to perform sample size calculations. The study was also underpowered to detect a difference between the split and 20∆ conditions, although the trend and visualization of the data strongly suggests a larger effect from split prisms.
Another important limitation is that there was no measurement of actual mobility with or without prisms, which must be done before any of the concepts put forward could be considered relevant to clinical practice. While a real-world relevance to collision judgment asymmetry is logical, there is essentially no prior studies to support this assertion. The only data available is from a small pilot study which found that patients with rightward collision judgment asymmetry walked more slowly in an obstacle course, but their walking path was not measured and they were not tested with prisms.28 Therefore, real-world relevance is anticipated but has yet to be proven. This is a critical step that must be taken to move the field forward towards having an effective evidence-based treatment for veering and imbalance caused by representational LVSN.
Future Directions
It is recommended that future studies first validate VR-measured collision judgment asymmetry by comparing the amount and direction of asymmetry to veering and obstacle passing distances during actual mobility. The mobility task could be walking; however, many in the target population require wheelchairs, making a wheelchair-driving task a preferable option as the primary outcome, with walking as a subpopulation secondary analysis. Subjects who are not normally utilizing a power wheelchair would need adequate training and practice time to acclimate prior to data collection. Eye and head tracking should be utilized to further improve understanding about which mobility errors are related to representational shifts from LVSN versus detection failures from homonymous field defects. A validated questionnaire such as the independent mobility questionnaire needs to be studied to determine if certain items are sensitive to the presence of representational errors. Lastly, additional pilot studies are needed to develop methods for prescribing and fitting split prisms, including dose finding and exploration of the use of frame customization with 3-D printing.
Conclusions
Split prisms improve the rightward collision judgment asymmetry in patients with LVSN after stroke, and results support online visual referencing of egocentric coordinates during the mobility-relevant task of collision judgment. Further studies are warranted to optimize design, develop prescribing methods, and evaluate real world effects, feasibility, and efficacy.
REFERENCES
- Stroke facts: Centers for Disease Control and Prevention; [cited 2025 April 16]. Available from: https://www.cdc.gov/stroke/data-research/facts-stats/index.html [
- Stroke Patients In The US: Understanding The Prevalence. 2025 [cited 2025 April 16]. Available from: https://medshun.com/article/how-many-stroke-patients-in-us
- Malhotra K, Gornbein J, Saver JL. Ischemic Strokes Due to Large-Vessel Occlusions Contribute Disproportionately to Stroke-Related Dependence and Death: A Review. Front Neurol. 2017;8:651-.
- Adair JC, Barrett AM. Spatial neglect: clinical and neuroscience review: a wealth of information on the poverty of spatial attention. Annals of the New York Academy of Sciences. 2008;1142:21-43.
- Buxbaum LJ, Ferraro MK, Veramonti T, Farne A, Whyte J, Ladavas E, et al. Hemispatial neglect: Subtypes, neuroanatomy, and disability. Neurology. 2004;62(5):749-56.
- Hedna VS, Bodhit AN, Ansari S, Falchook AD, Stead L, Heilman KM, et al. Hemispheric differences in ischemic stroke: is left-hemisphere stroke more common? J Clin Neurol. 2013;9(2):97-102.
- Chen P, Ward I, Khan U, Liu Y, Hreha K. Spatial Neglect Hinders Success of Inpatient Rehabilitation in Individuals With Traumatic Brain Injury: A Retrospective Study. Neurorehabilitation and neural repair. 2016;30(5):451-60.
- Barrett AM, Houston KE. Update on the Clinical Approach to Spatial Neglect. Current neurology and neuroscience reports. 2019;19(5):25.
- Chen P, Hreha K, Kong Y, Barrett AM. Impact of spatial neglect on stroke rehabilitation: evidence from the setting of an inpatient rehabilitation facility. Archives of physical medicine and rehabilitation. 2015;96(8):1458-66.
- Wee JYM, Hopman WM. Comparing consequences of right and left unilateral neglect in a stroke rehabilitation population. American Journal of Physical Medicine & Rehabilitation. 2008;87(11):910-20.
- Turton AJ, Dewar SJ, Lievesley A, O’Leary K, Gabb J, Gilchrist ID. Walking and wheelchair navigation in patients with left visual neglect. Neuropsychological rehabilitation. 2009;19(2):274-90.
- Dawson J, Thornton H. Can patients with unilateral neglect following stroke drive electrically powered wheelchairs? British Journal of Occupational Therapy. 2003;66:496-504.
- Qiang W, Sonoda S, Suzuki M, Okamoto S, Saitoh E. Reliability and validity of a wheelchair collision test for screening behavioral assessment of unilateral neglect after stroke. Am J Phys Med Rehabil. 2005;84(3):161-6.
- Watanabe S, Amimoto K. Generalization of prism adaptation for wheelchair driving task in patients with unilateral spatial neglect. Archives of physical medicine and rehabilitation. 2010;91(3):443-7.
- Huitema RB, Brouwer WH, Hof AL, Dekker R, Mulder T, Postema K. Walking trajectory in neglect patients. Gait & posture. 2006;23(2):200-5.
- Aravind G, Darekar A, Fung J, Lamontagne A. Virtual reality-based navigation task to reveal obstacle avoidance performance in individuals with visuospatial neglect. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2015;23(2):179-88.
- Buxbaum LJ, Palermo MA, Mastrogiovanni D, Read MS, Rosenberg-Pitonyak E, Rizzo AA, et al. Assessment of spatial attention and neglect with a virtual wheelchair navigation task. Journal of clinical and experimental neuropsychology. 2008;30(6):650-60.
- Aravind G, Lamontagne A. Perceptual and locomotor factors affect obstacle avoidance in persons with visuospatial neglect. Journal of neuroengineering and rehabilitation. 2014;11:38.
- Ogourtsova T, Archambault PS, Lamontagne A. Post-stroke unilateral spatial neglect: virtual reality-based navigation and detection tasks reveal lateralized and non-lateralized deficits in tasks of varying perceptual and cognitive demands. Journal of neuroengineering and rehabilitation. 2018;15(1):34.
- Punt TD, Kitadono K, Hulleman J, Humphreys GW, Riddoch MJ. From both sides now: crossover effects influence navigation in patients with unilateral neglect. Journal of Neurology, Neurosurgery & Psychiatry. 2008;79(4):464-6.
- Punt TD, Kitadono K, Hulleman J, Humphreys GW, Riddoch MJ. Modulating wheelchair navigation in patients with spatial neglect. Neuropsychological rehabilitation. 2011;21(3):367-82.
- Robertson IH, Tegner R, Goodrich SJ, Wilson C. Walking trajectory and hand movements in unilateral left neglect: a vestibular hypothesis. Neuropsychologia. 1994;32(12):1495-502.
- Tromp E, Dinkla A, Mulder T. Walking through doorways: An analysis of navigation skills in patients with neglect. Neuropsychological rehabilitation. 1995;5(4):319-31.
- Berti A, Smania N, Rabuffetti M, Ferrarin M, Spinazzola L, D’Amico A, et al. Coding of far and near space during walking in neglect patients. Neuropsychology. 2002;16(3):390-9.
- Ogourtsova T, Archambault P, Sangani S, Lamontagne A. Ecological Virtual Reality Evaluation of Neglect Symptoms (EVENS): Effects of Virtual Scene Complexity in the Assessment of Poststroke Unilateral Spatial Neglect. Neurorehabilitation and neural repair. 2018;32(1):46-61.
- Aravind G, Lamontagne A. Dual tasking negatively impacts obstacle avoidance abilities in post-stroke individuals with visuospatial neglect: Task complexity matters! Restorative neurology and neuroscience. 2017;35(4):423-36.
- Houston KE, Woods RL, Goldstein RB, Peli E, Luo G, Bowers AR. Asymmetry in the Collision Judgments of People With Homonymous Field Defects and Left Hemispatial Neglect. Investigative ophthalmology & visual science. 2015;56(6):4135-42.
- Houston KE. Collision judgments of patients with homonymous field defects (MSc Thesis). Boston, MA: New England College of Optometry; 2014.
- Wichmann FA, Jäkel F, Wixted J. Methods in psychophysics. Stevens handbook of experimental psychology and cognitive neuroscience. 2018;5:265-306.
- Bansal S, Han E, Ciuffreda KJ. Use of yoked prisms in patients with acquired brain injury: A retrospective analysis. Brain Injury. 2014;28(11):1441-6.
- Labreche T, Wild B, Dalton K, Leat SJ. Post‐stroke visual midline shift syndrome. Clinical and Experimental Optometry. 2020;103(3):290-5.
- Padula WV, Argyris S. Post trauma vision syndrome and visual midline shift syndrome. NeuroRehabilitation. 1996;6:165-71.
- Padula WV, Nelson CA, Padula WV, Benabib R, Yilmaz T, Krevisky S. Modifying postural adaptation following a CVA through prismatic shift of visuo-spatial egocenter. Brain Injury. 2009;23(6):566-76.
- Kraskin R. Lens Power in Action. 1983.
- Horner SH. The use of yoked prisms to enhance visual training. Optometric Extension Program. 1972;II.
- Kornheiser A. Adaptation to laterally displaced vision: a review. Psychological Bulletin. 1976;83(5):783-816.
- Rossetti Y, Rode G, Pisella L, Farne A, Li L, Boisson D, et al. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature. 1998;395(6698):166-9.
- Houston K, Woods R, Bowers A, Peli E. Split-prisms to improve collision judgments in left spatial neglect: A pilot study (Abstract). Optom Vis Sci. 2017;94(E-abstract 175046).
- Zhao H, Warren WH. On-line and model-based approaches to the visual control of action. Vision research. 2015;110(Pt B):190-202.
- Kohler I. The formation and transformation of the perceptual world. Psychological Issues. 1964;3:14-173.
- Pick HL, Jr., Hay JC, Martin R. Adaptation to split-field wedge prism spectacles. Journal of experimental psychology. 1969;80(1):125-32.
- Schenkenberg T, Bradforn DC, Ajax ET. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology. 1980;30:509-17.
- Gauthier L, Dehaut F, Joannette Y. The bells test: a quantitative and qualitative test for visual neglect. International journal of clinicial neuropsychology. 1989;11:49-54.
- Fenwick EK, O’Hare F, Deverell L, Ayton LN, Luu CD, McSweeney S, et al. Rasch analysis of the independent mobility questionnaire. Optometry and Vision Science. 2016;93(2):181-7.
- Houston KE, Paschalis EI. Feasibility of Magnetic Levator Prosthesis Frame Customization Using Craniofacial Scans and 3-D Printing. Translational vision science & technology. 2022;11(10):34.
- Jung J-H, Peli E. Impact of high power and angle of incidence on prism corrections for visual field loss. Optical Engineering. 2014;53(6).
- Qiu C, Jung J-H, Tuccar-Burak M, Spano L, Goldstein R, Peli E. Measuring pedestrian collision detection with peripheral field loss and the impact of peripheral prisms. Translational vision science & technology. 2018;7(5):1-.
- Houston KE, Peli E, Luo G, Bowers AR, Woods RL. Effects of Perceptual-motor Training on Collision Judgments with Peripheral Prism Expanded Vision. Optometry and Vision Science. 2022;99(12):875-84.
Conflicts: None
Funding: This material is the result of work supported with resources and the use of facilities at the VA Central Western Massachusetts Health Care System, Worcester, MA. Data collection was funded by Departmental Funds, Harvard Medical School, Department of Ophthalmology.
Acknowledgements: Eli Peli, OD, MSc, Alex Bowers, MCOptom, PhD, Robert Goldstein, PhD, Gang Luo, PhD, and Russell Woods, MCOptom, PhD for mentorship and development and maintenance of the virtual walking simulator used in the study.
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.