A Case Series of Ophthalmic Manifestations of Facial Botox Injections
ABSTRACT
BACKGROUND
Facial botulinum neurotoxin A (Botox) injections, one of the most performed cosmetic procedures in the United States, can lead to significant ocular complications. These adverse effects, such as acquired eyelid ptosis or double vision, can mimic ophthalmic manifestations of serious systemic conditions. Because of their growing popularity, complications of cosmetic procedures need to be incorporated into eye care providers’ differential diagnosis to most accurately examine and manage care for these patients.
CASE REPORT
Three patients, all with ocular adverse events secondary to botulinum neurotoxin facial injections, were examined to rule out more serious etiology of ptosis and esotropia. A recent history of these injections was not initially disclosed.
CONCLUSION
Some patients may hesitate to disclose their use of cosmetic injections or procedures due to worry of stigma or vagueness of questioning, or lack awareness of its relevance to their eye examination. The use of cosmetic procedures, such as Botox injections, should be inquired about with new onset ptosis or strabismus.
Keywords: Botox, botulinum neurotoxin, diplopia, ptosis
INTRODUCTION
Facial botulinum neurotoxin A (Botox) injections are one of the most performed cosmetic procedures in the United States because of their ability to provide smooth, wrinkle-free skin. Botox injections for treatment of facial rhytids (wrinkles), especially to the upper one-third of the face, typically provide high patient satisfaction and relatively predictable outcomes.1 While this may be the case for most patients, several ocular complications may arise after treatment.1,2
Botulinum neurotoxin (BoNT) is an endotoxin produced by the bacterium Clostridium botulinum. It blocks the release of neurotransmitter acetylcholine at the neuromuscular junction, decreasing contraction of the muscle where it is introduced.3 The toxin binds irreversibly within hours to the presynaptic neuron, and the full effect may not be complete for two weeks. This causes a breakdown of the neuromuscular junction, leading to muscular paralysis. Due to ongoing turnover, muscular function returns approximately three months after treatment and is usually complete by six months after treatment.4 The neurotoxin exists in seven immunologically distinct forms, serotypes A through G, but only two serotypes have been approved for clinical use.5 Botulinum toxin serotype A (used in Botox Cosmetic) is primarily used for cosmetic treatment and serotype B is primarily used for medical conditions such as dystonia.1
The muscles of facial expression are unique as they have soft tissue attachments to skin, unlike most muscles which have bony attachments.1 The formation of facial wrinkles is due to repetitive contraction of this underlying musculature. Injecting smaller quantities of the botulinum neurotoxin into areas of interest results in contraction weakness or paralysis of these overactive muscles, resulting in flattening of the overlying skin and reduced appearance of wrinkles.1,4 The U.S. Food and Drug Administration (FDA) first approved use of botulinum toxin for cosmetic use in 2002 as Botox to treat glabellar complex muscles that form frown lines and in 2013 to treat lateral orbicularis oculi muscles that form crow’s feet.1,4 Botox use for other cosmetic procedures is currently off-label. Botox has become the treatment of choice for wrinkles occurring in the upper one-third of the face. Other indications for botulinum toxin use include blepharospasm, strabismus, cervical dystonia, migraines, hyperhidrosis, muscle spasticity and more.1,5
Most cosmetic applications of Botox involve the upper face for aesthetic treatment.2 This includes the frontalis, glabellar complex (corrugator supercilii, depressor supercilii, procerus, and medial fibers of orbicularis oculi), orbicular oculi, and nasalis muscles.1,6 Injection of BoNT into these locations will reduce the appearance of forehead lines, frown lines, crow’s feet, and bunny lines, respectively (see figure 1). Treatment of these areas may lead to ocular and periorbital complications due to proximity and shared vasculature.
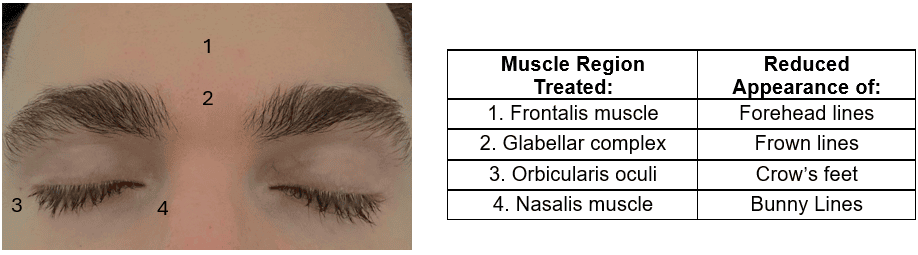
Figure 1. The muscle region treated with botulinum neurotoxin to reduce the appearance of facial wrinkles in each corresponding region. *This patient gave informed consent to publish this photo.
Complications that may present after injection of the neurotoxin to the upper face may include ptosis of the upper eyelid or eyebrow, strabismus, ocular surface disease, ectropion, and lagophthalmos.2,7 Acute onset acquired signs like these, especially eyelid ptosis and strabismus, can be concerning for both the patient and provider due to the myriad of other systemic or neurological etiologies.8,9 This case series displays clinical presentations of adverse events post facial treatment with botulinum neurotoxin. At times, patients may not be forthcoming about their use of cosmetic injections with BoNT, making it important to keep this on the list of differentials prior to urgent systemic or neurological workup.
CASE SERIES
Case 1
A 47-year-old male presented for evaluation of new onset, intermittent, horizontal diplopia, worse in the distance that started approximately one week prior. The diplopia disappeared when either eye was covered and he only noticed the diplopia at distance and intermediate range, but not at near. He denied recent trauma or associated headaches, lethargy, unsteadiness, and dizziness along with the diplopia. He denied recent hiking or skin rashes, any difficulty breathing or swallowing, weakness in his extremities, lid drooping or pain on eye movement. He reported a mild right-sided earache that started the day prior to the appointment but denied any tinnitus or whooshing sounds.
The patient had a pertinent ocular history of self-reported “lazy eye” as a child with a history of vision therapy. He was unsure if the lazy eye was due to strabismus or refractive error (mild anisometropia suggested history of mild refractive amblyopia). Pertinent medical history included hypercholesterolemia controlled with oral atorvastatin 10 mg daily. His last medical exam was approximately one month prior to the appointment with full routine blood work and a sexually transmitted disease panel, which were all unremarkable per patient report. The patient was a fitness trainer by occupation.
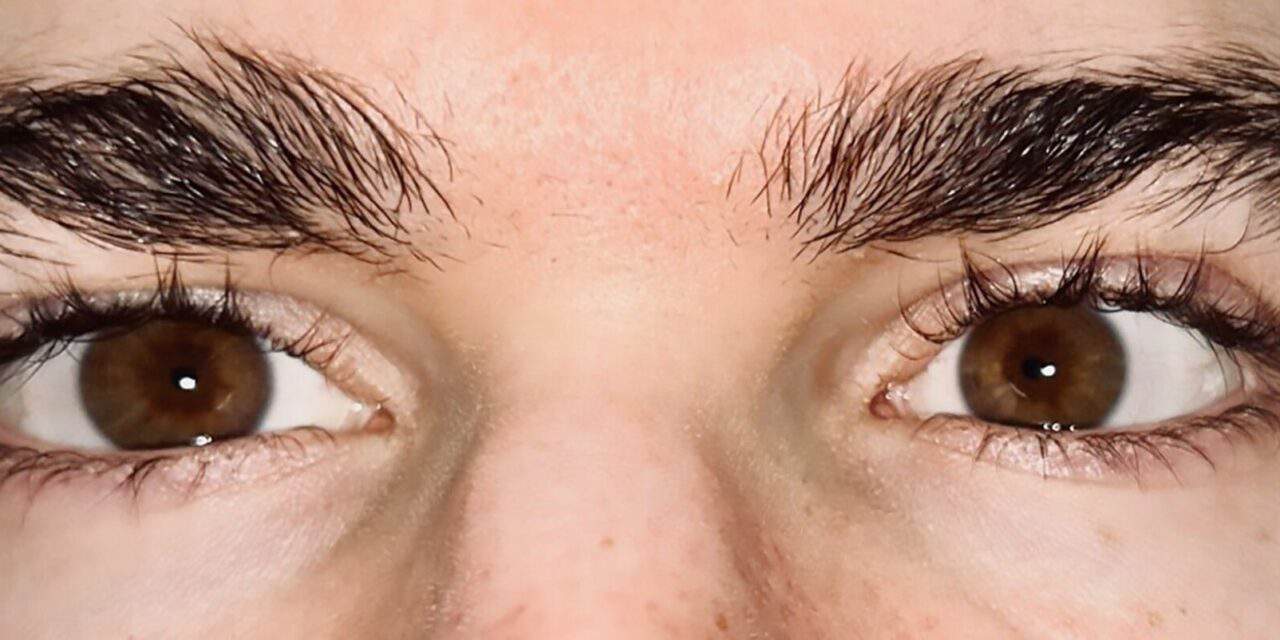
His entering Snellen acuity with habitual spectacle correction was 20/15 in each eye. Pupils were equally round and reactive to light without an afferent pupillary defect. His extraocular motilities were full in all gazes with mild saccadic undershoot in all gazes. There was no fatigue noted with prolonged gaze superiorly. Distance cover test with correction showed a constant alternating, but primarily left 6 prism diopter esotropia and near cover test with correction showed an 8 prism diopter esophoria (Figure 2). Deviation in nine positions of gaze at near demonstrated a comitant 13 prism diopter eso deviation and 1 prism diopter right hyper deviation.
Figure 2. Small angle esotropia while viewing a distance target. *This patient gave informed consent to publish this photo.
Slit lamp examination was unremarkable with no signs of anterior segment inflammation. Intraocular pressures by Goldmann applanation tonometry were 20 mmHg in each eye. Funduscopic examination demonstrated an absence of disc edema or hemorrhages in either eye and healthy-appearing optic nerves, vasculature and maculae in each eye.
Due to the comitant and alternating nature of the esotropia at distance, urgent neurological workup was not indicated. The patient also had low systemic risk factors for ischemic etiologies suspicious for ischemic sixth nerve palsy, had recent blood work that was unremarkable for abnormal thyroid function or sexually transmitted infection, and no recent history of trauma. The patient was referred to their primary care provider to request a baseline MRI of the brain, orbits, and sixth cranial nerve pathway as well as blood work to rule out potential systemic infectious, inflammatory or autoimmune etiologies. To reduce patient symptoms, monocular occlusion with Bangerter foil or tape was recommended due to fluctuating symptoms and esotropia.
The patient returned after receiving an unremarkable MRI and blood work. Upon further discussion, the patient had mentioned that he had received facial Botox injections a few weeks prior to the onset of the new diplopia. Due to the exam findings and newly disclosed history of Botox injections, this was determined to be the most likely underlying cause of the new-onset esotropia.
Case 2
A 42-year-old female presented for evaluation of a new constant right eyelid droop for five days (Figure 3). She denied associated symptoms including double vision, headache, pain, redness, itching and or eye rubbing. The patient denied worsening or fluctuations in the severity of ptosis throughout the day. She denied any history of contact lens wear or trauma. Medical history was unremarkable. She reported no allergies and was using artificial tears occasionally for dry eye symptoms.

Figure 3. Photo of the patient’s asymmetric lid position and ptosis of the right upper eyelid (patient looking slightly to her left). *This patient gave informed consent to publish this photo.
Her uncorrected entering visual acuities were 20/20 in the right and left eye. The pupils were equally round and reactive to light with no afferent pupillary defect noted in either eye. Her extraocular motilities were full in all gazes with no diplopia reported and no lid fatigue noted with prolonged upgaze. The distance and near uncorrected cover test was orthophoric. The margin to reflex distance (MRD1) was 2 mm in the right eye and 4 mm in the left eye, indicating a 2 mm ptosis of the right upper eyelid (Figure 3). The slit lamp examination revealed mild meibomian gland dysfunction in both eyes and reduced tear break up time, otherwise unremarkable with no signs of anterior segment inflammation. Intraocular pressures by Goldmann applanation tonometry were 20 mmHg in each eye. Funduscopic examination of the posterior segment was unremarkable. No mechanical cause of ptosis was identified, and no signs or history of trauma were apparent. Some differential diagnoses considered were aponeurotic (involutional), neurologic, myogenic and autoimmune. Age, trauma, ocular surgery, chronic eye rubbing, longstanding use of rigid gas permeable lens or use of chronic steroid may lead to aponeurotic defect-related acquired ptosis, all of which were ruled out based on the case history. Upon further questioning, the patient reluctantly reported Botox injections of her forehead and glabellar region for cosmetic purposes approximately one week prior. This was identified to be the most likely cause of the new onset ptosis.
Case 3
A 38-year-old female presented with new, sudden-onset, constant drooping of her left upper eyelid that started approximately a week prior. She denied fluctuation or worsening in severity throughout the day as well as any recent ocular injury, surgery or infection. She reported no change in vision, ocular pain, redness, burning, itching, or tearing. The patient denied double vision, headaches, vertigo, dizziness, generalized muscle weakness or difficulties with breathing or swallowing. She had no pertinent ocular history and her medical history was remarkable for borderline high cholesterol managed with lifestyle modifications. She denied the use of any medications and allergies.
Her entering uncorrected visual acuity was 20/20 in each eye. Her extraocular motilities were smooth and full in both eyes, saccades were full in both eyes, and the near point of convergence was to the nose. Uncorrected distance and near cover test revealed an orthophoric posture. Her confrontation visual fields were full to finger count in both eyes, and pupils were equally round and reactive to light without an afferent pupillary defect in either eye.
Her levator function measured 18 mm and 17 mm in the right and left eye respectively. Her margin reflex distance (MRD1) for the right eye was 4.5 mm and the left eye was 3.5 mm, indicating a 1 mm ptosis of the left upper eyelid. Her vertical palpebral aperture in the right eye was 10 mm and the left eye was 8.5 mm. Upper eyelid crease for the right eye was noted to be at 4 mm and the left eye was at 4.5 mm. No lagophthalmos was noted in either eye and when the left eyelid was manually lifted, no drooping of the right upper eyelid was noted. There was no eyelid retraction or lid lag noted for either eye. The lid fatigue test was negative in prolonged upgaze. Of note, when the patient was asked to raise her eyebrows there was negligible change in the position of either eyebrow with minimal to no contraction of the frontalis muscle. Her eyebrows appeared to be tattooed and when asked to furrow her eyebrows, there were no rhytids or wrinkles noted on either side of the forehead. The forced lid closure test was remarkable for weak orbicularis oculi strength on both sides. However, the patient was able to puff out her cheek and smile symmetrically without difficulties. Facial sensation was noted to be equal on both sides of the face and the patient noted equal hearing sensation to finger rub in front of each ear.
Lids were everted on slit lamp exam without remarkable findings. Her intraocular pressures with Goldmann applanation tonometry were 17 mmHg in the right eye and 18 mmHg in left eye. The dilated fundus exam was unremarkable in both eyes. Upon further questioning specifically related to tattooed eyebrows and the possibility of cosmetic procedures, she acknowledged having Botox injection a couple of weeks prior to onset of unilateral left upper eyelid ptosis, which was deemed the most likely cause for her ptosis.
DISCUSSION
An acute onset of acquired unilateral ptosis or esotropia requires careful examination to rule out more concerning etiologies. In cases of new-onset esotropia, sixth nerve palsies secondary to ischemia, vasculopathy, trauma, infection (such as Lyme disease), autoimmune disease, elevated intracranial pressure, and neoplasm must be ruled out in adult patients.8 In cases of new-onset unilateral lid ptosis, neurogenic causes, most commonly third nerve palsies or Horner syndrome, as well as myogenic causes such as myasthenia gravis must be considered.9,10
In the setting of acute-onset acquired ptosis, if no mechanical cause of an acquired ptosis is identified and no signs or history of trauma are apparent, differentials to consider include are aponeurotic (involutional), neurologic, myogenic and autoimmune-related.9 Age, trauma, ocular surgery, chronic eye rubbing, longstanding use of rigid gas permeable lens or chronic use of steroid may lead to aponeurotic defect related acquired ptosis, which can be primarily identified during case history.10 In the absence of anisocoria and ocular motility dysfunction, cranial nerve three palsy and Horner’s syndrome can typically be successfully ruled out. Weakness of the orbicularis oculi and frontalis muscle in the setting of normally functioning lower 2/3rd of the facial muscles, such as in case 3, supports non-involvement of cranial nerve seven and should increase suspicion of a possible history of recent facial BoNT use for cosmesis.
For a patient presenting with an isolated acute-onset acquired esotropia, traumatic and iatrogenic causes will be ruled out during the case history. Vasculopathic risk factors, such as diabetes, hypertension and atherosclerosis increase suspicion for ischemic sixth nerve palsies. Infectious etiologies, such as syphilis or Lyme disease, should also be considered depending on sexual history and region, as well as autoimmune causes, such as thyroid eye disease; all of which can be ruled out with prompt blood work. Neurological imaging may be indicated in patients under 50 in the absence of vasculopathic risk factors in combination with bloodwork to rule out neoplasia or increased intracranial pressure.8
Myasthenia gravis causes immune-mediated damage to acetylcholine receptors and is the most common cause of ptosis and strabismus from dysfunction at the level of the neuromuscular junction.8,10 This would typically be associated with generalized muscle weakness, difficulties breathing or swallowing, and worsening or fluctuating symptoms throughout the day. Sub-acute to acute unilateral upper eyelid ptosis or strabismus in the absence of generalized muscle weakness may help to rule out myogenic causes like chronic progressive external ophthalmoplegia, myotonic dystrophy and oculopharyngeal dystrophy.
In all three cases, the use of facial Botox injections was identified as the etiology for the acquired strabismus or upper eyelid ptosis. Undesired effects like these may occur approximately two weeks after treatment and occur if the toxin diffuses into nearby musculature.1,2,7 Temporary eyelid ptosis after use of facial Botox is uncommon (1-5%), almost always unilateral, seen as 2-3 mm lowering of the affected eyelid, can be worse at the end of the day due to muscle fatigue, and the most common complication in the treatment of the glabellar complex.1,11 This happens due to migration of the neurotoxin through the orbital septum fascia to the levator palpebrae superioris, which elevates the upper eyelid.1 Similarly, the neurotoxin can diffuse into the orbit and affect the extraocular muscles, which can lead to strabismus. This is a rarer complication (approximately 2%) and typically affects the lateral rectus muscle, especially when treating crow’s feet, as the injection site is the temporal orbicularis oculi muscle.2,12
The incidence of these complications declines as injector skill improves because technique and experience play an important role in reducing these risks.1 Incidence of ptosis and strabismus can be reduced by adjusting injection site further from orbital rim when treating the glabellar region as well as maintaining appropriate angle and direction of the injection.1,12 Patients are also often advised to avoid lying supine and avoid massaging or applying heat to treatment areas on the day of treatment to reduce the potential spread of the toxin.1
Management of these side effects is typically conservative, as they are temporary and resolve as the neurotoxin wears off within a couple of weeks to months.1,2,12 Cosmesis of the ptosis may be treated with various ophthalmic solutions, including over-the-counter naphazoline 0.025%/pheniramine 0.3%, prescription apraclonidine 0.5% or oxymetazoline hydrochloride 0.1% (Upneeq). Their alpha-adrenergic effects will lead to contraction of the Müller muscle, elevating the lid approximately 1-2 mm.1,11 For acquired strabismus post-Botox, eye patching or use of Fresnel prism on habitual correction can help with patient symptoms and comfort until resolution of the diplopia.11 In case 1, the patient was recommended to use Bangerter occlusion foil over non-dominant eye as his double vision was primarily in the distance and fluctuated throughout the day. In the second case, the patient was treated with oxymetazoline hydrochloride 0.1% ophthalmic solution once daily into the right eye. This successfully improved the cosmesis of the right upper eyelid ptosis by lifting the lid approximately 2 mm, and repeat MRD1 testing showed 4 mm in each eye. In case 3, the patient deferred treatment for the left upper eyelid ptosis, knowing that the effect would wear off in approximately a three-month period.
CONCLUSION
Given the increased popularity and accessibility of facial botulinum neurotoxin treatments, especially among younger age groups, it is important for eye care providers to be aware of the potential associated ophthalmic complications which have been demonstrated in each of these cases. With the increasing popularity of cosmetic procedures, such as Botox injections, clinicians should inquire about recent cosmetic treatments and consider them in the list of differential diagnoses.
REFERENCES
- Small R. Botulinum toxin injection for facial wrinkles. Am Fam Physician. 2014;90(3):168-175.
- Borba A, Matayoshi S, Rodrigues M. Avoiding Complications on the Upper Face Treatment With Botulinum Toxin: A Practical Guide. Aesth Plast Surg. 2022;46(1):385-394. doi:10.1007/s00266-021-02483-1
- Lagueny A, Burbaud P. Mécanisme d’action, indication et résultats des traitements par la toxine botulinique. Neurophysiologie Clinique/Clinical Neurophysiology. 1996;26(4):216-226. doi:10.1016/S0987-7053(96)85003-9
- Klein AW. Contraindications and complications with the use of botulinum toxin. Clinics in Dermatology. 2004;22(1):66-75. doi:10.1016/j.clindermatol.2003.12.026
- Bakshi E, Hartstein ME. Compositional differences among commercially available botulinum toxin type A: Current Opinion in Ophthalmology. 2011;22(5):407-412. doi:10.1097/ICU.0b013e328349b0b6
- Walker HM, Chauhan PR. Anatomy, Head and Neck: Glabella. [Updated 2023 Jul 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
- Bonińska K. Ophthalmic Complications after Needle-Based Medical Aesthetic Procedures: A Narrative Review. JCM. 2022;12(1):313. doi:10.3390/jcm12010313
- Graham C, Gurnani B, Mohseni M. Abducens Nerve Palsy. [Updated 2023 Aug 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482177/.
- Shahzad B, Siccardi MA. Ptosis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK546705/.
- Bhatti MT. Basic and Clinical Science Course (BCSC), section 05: Neuro-Ophthalmology. In: ; 2020.
- Witmanowski H, Błochowiak K. The whole truth about botulinum toxin – a review. Postepy Dermatol Alergol. 2020;37(6):853-861. doi:10.5114/ada.2019.82795
- Delle Chiaie T. Diplopia Secondary to Neurotoxin Injections: Prevention, Diagnosis, and Management. J Clin Aesthet Dermatol. 2024;17(3-4 Suppl 1):S30-S36.