
Charles Bonnet Syndrome Precipitated by Brimonidine Tartrate
ABSTRACT
BACKGROUND
Charles Bonnet Syndrome (CBS) induced by brimonidine tartrate ophthalmic solution has rarely been reported. To the best of the author’s knowledge, there are six previously reported cases in the literature. CBS is a condition characterized by visual hallucinations in patients with vision loss and clear cognition. This condition is more prevalent in the elderly population, between the ages of 75 and 84 years old. CBS is likely under-reported due to the reluctance of patients to report the condition. With increases in life expectancy, eye care providers will be seeing more patients with visual impairment who are at risk of developing CBS. This article reports a case of CBS induced by brimonidine tartrate ophthalmic solution in an 88-year-old Hispanic male.
CASE REPORT
An 88-year-old Hispanic male presented to the emergency eye clinic with complaints of visual hallucinations that started two days after the initiation of brimonidine tartrate 0.2% ophthalmic solution for glaucoma. The hallucinations included images of a ghost, and sometimes, a plant to his right side. He knew these images were not real and were not causing him alarm. The patient had a complex ocular history which included primary open angle glaucoma (POAG) in both eyes (OU), Fuchs endothelial dystrophy OU, pseudophakia OU, cystoid macular edema OU, and was status post membrane peel for epiretinal membrane OU. His best corrected visual acuity was 20/200 in the right eye (OD) and 20/60 in the left eye (OS). There was near total visual field loss OD and 40 degrees of central field remaining OS. He did not have acute changes in his ocular conditions at the exam. The patient was advised to discontinue the brimonidine eye drops, and the visual hallucinations resolved within two to three days.
CONCLUSION
With increases in life expectancy and improvements in access to medical care, eye care professionals are likely to see more patients with visual impairment. As front-line eye care providers, optometrists should be aware of Charles Bonnet Syndrome and recognize the association of brimonidine tartrate ophthalmic solution and visual hallucinations in cognitively intact individuals. Treatment should be provided to optimize the patient’s vision and ocular health, and providers should make appropriate referrals to mental health professionals if the visual hallucinations are causing the patient distress or do not fit the characteristics of Charles Bonnet Syndrome. Eye care providers can play a critical role in reassuring patients and their families that the visual hallucinations of CBS do not indicate a cognitive decline. Furthermore, eye care providers are encouraged to rise to the challenge of talking to visually impaired patients about the potential for CBS, especially if initiating brimonidine therapy.
Keywords: Charles Bonnet Syndrome, visual hallucination, brimonidine tartrate
INTRODUCTION
Charles Bonnet Syndrome (CBS) is a medical condition in which complex visual hallucinations occur in the setting of visual impairment and preserved cognition.1-4 CBS patients are usually between 75 and 84 years of age.1 The prevalence of CBS varies in the literature and ranges from 0.4% to 30%.1,3,5-7 Literature suggests that CBS is vastly under-reported, mainly due to the fear of being regarded as having mental instability in the elderly population.1,3,6
The pathophysiology of CBS starts with any eye disease that leads to vision loss. These conditions can include cataracts, glaucoma, diabetic retinopathy, age-related macular degeneration, or cerebral infarction affecting the visual cortex.8 The visual hallucinations experienced in CBS usually consist of human figures, faces, animals, plants, and buildings.1,3,6,9,10 These hallucinations can last between seconds to hours, frequently with color and movement.2 The visual hallucinations are not associated with any other type of sensory hallucination (i.e. auditory) and the patient is aware of its unreal nature.1,2,3,6
Alpha-agonists, including oral clonidine and topical brimonidine, can cause neuropsychiatric side effects because of their ability to cross the blood-brain barrier.8 Brimonidine ophthalmic solution is a topical alpha-agonist, which is commonly used to treat ocular hypertension and glaucoma.8 It can cause neuropsychiatric side effects, albeit at a lower rate than clonidine due to its reduced lipophilic properties.8 There have been reported cases of brimonidine induced encephalopathy, psychosis, and CBS.11 The prevalence of brimonidine-induced CBS is rare. Only six cases have been previously reported.
There is a lack of standardized treatment options for CBS in ophthalmic literature. Management strategies are targeted to maximize the patient’s vision through spectacles, contact lenses, optical aides, and/or low vision rehabilitation.3 Pharmacotherapy with antipsychotics have been found to have mixed efficacy in the treatment of CBS.3 Surgical intervention may be warranted when the pathology can be treated with a resultant anticipated improvement in vision.3 One of the most important elements in managing CBS is educating and counseling the patient and their family members about the condition and providing reassurance that this is not a sign of cognitive decline.3 It is important for eye care providers to be able to recognize CBS and the potential for brimonidine to potentiate CBS.
CASE REPORT
An 88-year-old Hispanic male presented to the eye clinic for a follow up examination. He had a complex ocular history which included: basal cell carcinoma of the right lower eyelid, Fuchs endothelial dystrophy in both eyes (OU), hypertensive retinopathy OU, pseudophakia OU, cystoid macular edema OU, history of membrane peel for epiretinal membrane OU, advanced primary open angle glaucoma in the right eye (OD), moderate primary open angle glaucoma in the left eye (OS), and intraocular pressure increase on steroid therapy. He wore polycarbonate bifocal glasses full time. The patient had misplaced the latanoprost and dorzolamide/timolol eye drops a few days prior to the examination and had used prednisolone acetate 1% ophthalmic suspension (from a previous prescription) twice a day (BID) OU instead. His systemic history included hypertension and hyperlipidemia. He was taking lisinopril, hydrochlorothiazide, and rosuvastatin for more than 10 years. He was also taking vitamin D and aspirin for an unknown length of time. He had no known allergies. He lived with his daughter, who accompanied him to his medical exams. He was oriented to person, place, and time.
At this presentation, his entering visual acuity with spectacle correction was 20/200 OD and 20/60 OS. Confrontation visual fields were constricted in all quadrants OD and superiorly OS due to glaucoma. There was an afferent pupillary defect OD. Slit lamp examination showed stromal corneal edema and endothelial guttata OU. Intraocular pressures (IOP) with Goldmann applanation tonometry were 50 mmHg OD and 24 mmHg OS. Gonioscopy showed open angles without neovascularization or peripheral anterior synechiae OU. The IOP was lowered in the right eye in-office to 31mmHg. Dilated fundus examination revealed a cup-to-disc ratio of 0.90 round OD and 0.65 round OS. The optic nerves showed diffuse pallor and peripapillary atrophy OU. There was macular puckering OU. The retinal vessels were tortuous OU. The patient was instructed to discontinue using the prednisolone acetate 1%, restart dorzolamide/timolol BID OU and latanoprost QHS OU, and start brimonidine BID OD. He had an appointment scheduled with his retina specialist in two weeks, so his retina specialist was alerted and agreed with the plan.
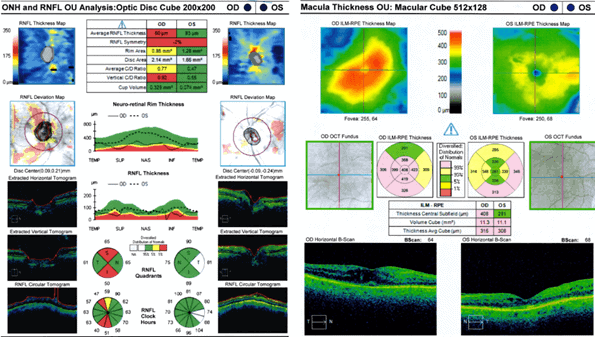
Ocular imaging was not performed at this examination. Optical coherence tomography (OCT) of the retinal nerve fiber layer (RNFL) and macula shown in Figure 1 were performed 6 months prior to this visit. The OCT RNFL showed thinning of the superior and inferior quadrants OD (Figure 1a). The OCT macula showed cystoid macular edema OU, worse OD than OS (Figure 1b). The Humphrey Visual Field (HVF) was performed 2 months prior (Figure 2). The records from his glaucoma provider were not available for review.

Figure 1. Left image: OCT RNFL shows cupping and RNFL thinning OD>OS. Right image: OCT macula shows cystoid macular edema OD>OS. Both OCT images were taken six months prior.

Figure 2. HVF 24-2, performed 2 months prior, shows severe visual field constriction OD. There was an altitudinal defect with near complete loss of superior visual field and inferior arcuate defect OS.
Five days later,he patient presented to the emergency walk-in eye clinic due to new onset visual hallucinations. He was accompanied by his daughter who suspected the hallucinations may be related to the brimonidine tartrate 0.2% ophthalmic solution. The symptoms began two days after initiating treatment. The hallucinations included images of a ghost and, sometimes, a plant to his right side. He noticed the images frequently throughout the day. They disappeared with blinking and with changing his fixation. He stated that he knew these images were not real and they were not causing him alarm. He denied auditory hallucinations. He did not have a history of psychological disorders and denied having experienced these visual hallucinations in the past. The patient’s daughter denied recent behavioral or mood changes in the patient. Other than starting brimonidine tartrate 0.2% ophthalmic solution, there were no changes to his medical history since the previous exam. He was oriented to person, place, and time. His IOP was 7 mmHg OD and 15 mmHg OS with Goldmann applanation tonometry. Dilated fundus examination was unchanged compared to the previous exam. He was advised to discontinue the brimonidine tartrate 0.2% ophthalmic solution and to continue the dorzolamide/timolol ophthalmic solution twice daily OU and latanoprost QHS OU. He was counseled to continue wearing his spectacle correction full time. The patient was to continue care with his retina specialist and glaucoma specialist. He was not referred to the mental health clinic or neurology as there was no cognitive or behavioral concern from him and his daughter. He was instructed to report to the emergency room or the acute mental health clinic if he or his family members noticed changes in behavior.
At his follow up with the retina specialist, he reported resolution of the visual hallucinations within two to three days of suspending brimonidine. At this visit, his best corrected visual acuity was stable at 20/200 OD and 20/60 OS. IOP with Goldmann applanation tonometry were 12 mmHg OD and 15 mmHg OS. There were no acute changes in the dilated fundus examination. The OCT macula (Figure 3) and HVF 24-2 OS (Figure 4) were stable as well. HVF OD and OCT RNFL were not performed on this follow-up visit.
Given the temporally associated nature of the visual hallucinations with the initiation and cessation of brimonidine tartrate ophthalmic solution, the patient was diagnosed with brimonidine-induced Charles Bonnet Syndrome. At the one year follow up, the patient was cognitively intact and without signs of dementia. He remained free of visual hallucinations. His glaucoma medications were changed to pilocarpine 1% three times a day OU, netarsudil-latanoprost before bedtime OU, and dorzolamide three times a day OU. He continues his eye care with his retina specialist and glaucoma specialist.
DISCUSSION
Charles Bonnet Syndrome (CBS) is characterized by complex visual hallucinations in a visually impaired individual without cognitive dysfunction.1,2,3,5,9 Depending on the study, the prevalence of CBS varies and ranges from 0.4% to 30%.1,3,5-7 CBS is more common in the elderly between the ages of 75 and 84 years due to the higher incidence of profound vision loss with age.1,6 The prevalence varies because the diagnosis of the condition requires the patient to self-report the visual hallucinations. The visual symptoms are believed to be under-reported by the elderly population due to fear of being labeled mentally unstable by their loved ones or healthcare professionals.1,2,3,6 This age group overlaps with those at risk of developing dementia, so the etiology of the visual hallucinations can be challenging to distinguish between CBS and early dementia.11
A major limitation in studying the collective data on CBS lies within the definition, or lack thereof. Without a standardized definition, diagnostic criteria vary amongst medical specialties.1,3 Unlike eye-specific conditions that are diagnosed primarily by eye care providers, CBS can be diagnosed across multiple specialties including optometrists, ophthalmologists, neurologists, and psychiatrists. Most of the information on CBS is reported in psychiatric and neurologic literature, rather than optometric and ophthalmologic literature. In 1989, psychologists Gold and Rabins developed a diagnostic criteria of CBS which is commonly cited in psychiatric literature and includes:12
- The presence of formed, complex, persistent or repetitive, stereotype visual hallucination;
- Full or partial retention of insight;
- Absence of primary or secondary delusions; and
- Absence of hallucinations in other modalities
It is important to note that visual impairment was not a criterion of diagnosis according to Gold and Rabins. Others, including psychiatrist Tuenisse et al., have proposed criteria that also does not include visual impairment as a critical component in diagnosis of CBS.2 In contrast, eye care providers consider visual impairment a key criterion in the diagnosis of CBS. Many investigators report a strong association between CBS and visual impairment, however there is no consensus on whether visual pathology and dysfunction is mandatory for the diagnosis of CBS. Neither the American Academy of Ophthalmology nor the American Academy of Optometry have established diagnostic criteria for CBS.
CBS has been documented in the context of visual impairment due to pathology anywhere along the visual pathway, from the eye to the occipital cortex.1 The ophthalmologic diagnostic criteria of CBS does not establish a quantitative minimum visual acuity or visual field constriction. The most common ocular pathology associated with CBS is age-related macular degeneration.1 Gilmour et al. found that central visual acuity of worse than 20/40 in the better seeing eye was found to be associated with significant risk of developing visual hallucinations.7 CBS occurred in their low vision patients with visual acuity of 20/40 to 20/1600.7 Subjects were twice as likely to have CBS when their visual acuity was 20/300 or worse.7 CBS is more likely to occur in patients with higher degrees of bilateral visual impairment, especially in the context of sudden reduction in visual function.1 Reports suggest that improvement in visual function, spontaneously or by intervention (such as cataract surgery) results in improvement, or complete cessation, of CBS.1 Spontaneous cessation of CBS in the setting of progressive vision loss has been seen in some patients, suggesting that the hallucinations may be unrelated to the degree of visual impairment.1
CBS can occur in the setting of peripheral vision loss with preserved central vision.3,9 Bilateral advanced visual field defects caused by glaucoma, homonymous hemianopia due to stroke, and even bitemporal field defect due to pituitary adenoma can result in CBS.13,14 The correlation between visual field loss and incidence of CBS has not been well studied. There are limited studies that have investigated the correlation between visual field loss and report the prevalence of CBS to range from 2.8% to 13.5%.9 Peters et al. found that visual acuity ranged from 20/20 to 20/40 with extensive visual field defects in glaucoma patients who reported CBS.9 They also found that glaucoma patients with CBS were more likely to have visual field index of <30% in at least one eye.9 The correlation between the location of the visual hallucination and visual field loss remains controversial. Some reports suggest that complex visual hallucinations have a predilection for the area of visual field loss.1 However, other investigators found contradictory evidence that there is no relationship between the location of the visual hallucination and scotomatous visual field.1
The visual hallucinations experienced in CBS may be simple or complex. Simple visual hallucinations include simple shapes, grid-like patterns, and branching patterns.1,2,3,6,9,10 Complex visual hallucinations are complicated images of people, faces, animals, flowers, trees, and plants. 1,2,3,6,9,10 The hallucinations are usually well organized, defined, more vivid, and clear than the individual’s normal functional vision.1,15 The hallucinations may contain familiar and unfamiliar images, occur in color or black and white, be motionless or show movement, and/or fit well into the surroundings or be randomly projected in relation to the surroundings.2,3 Episodes can occur multiple times a day to only twice a year.2 These hallucinations typically occur without triggering factors.1,16 However, in some individuals, triggers may include: fatigue, stress, dim or bright illumination, or even social isolation.1,7,12 These hallucinations can be recurrent and persistent and last from seconds to hours.1,2,6 The characteristics of the hallucinations can vary with each episode.1,2,4 Unlike visual hallucinations experienced in patients with dementia, individuals with CBS maintain “insight” of the unreal nature of the hallucinations and can describe their visual hallucinations in detail.1,2,3,6 In one study, 87% of patients recognized the hallucinations were not real after the first episode.7
CBS patients usually respond to the visual hallucinations in a neutral manner without emotional distress because they retain insight to the illusory nature of the hallucinations.7,12,13,15 While their reactions can vary from indifference to irritation and fear, the hallucinations are usually pleasant.1,2,12 This is in contrast to patients with dementia who experience visual hallucinations.1,2,12 Only one-third of patients with dementia realize they are hallucinating, with greater cognitive impairment associated with poorer insight.17 Approximately 50% of dementia patients are significantly distressed by their visual hallucinations, with fear and anger being the most common responses.17
The true mechanism of visual hallucinations that occur in CBS has not been established.5 The most popular theory is the deafferentation theory, also known as the sensory deprivation theory.3,18 The theory hypothesizes that reduced visual acuity or visual field defects decreases input to the visual cortex, which increases spontaneous neuronal discharge to the visual cortex.3 First, there is an increase in the number of neurotransmitters released in the presynaptic neuron.3 Second, there is an increased number of receptors in the postsynaptic membrane caused by prolonged inactivity, which leads to an increase in the intensity of response to the neurotransmitters released.3 Last, there is hyperexcitability of neurons due to the changes in the amount of gamma-aminobutyric acid and glutamatergic N-methyl-D-aspartic acid within the synapse.3 This spontaneous excitability of neurons can result in visual hallucinations.3,4,19 Functional MRI (fMRI) studies have supported the deafferentation theory by confirming the visual cortex activation that results in visual hallucinations in visually impaired patients.5,16 These studies also showed that the neuronal input into the visual cortex that leads to spontaneous discharge is absent in patients with congenital blindness.1 This supports the fact that visual hallucinations are not reported in patients with congenital blindness.4
Alpha-2 agonists, such as clonidine and brimonidine, can cross the blood brain barrier and stimulate the central nervous system (CNS) alpha-adrenoreceptors, resulting in hypotension, bradycardia, and sedation.8 Clonidine is an oral alpha-2 agonist medication used in the treatment of systemic hypertension.20,21 Central nervous system side effects include depression, acute visual and auditory hallucinations, and even delusional psychoses.20,21,22 Brown et al. described the case of a 31-year-old woman treated with clonidine for poorly controlled hypertension who developed vision of “hands reaching for her from a closet or an old woman following her” in the presence of an otherwise clear sensorium.20,22 He described two similar cases of oral clonidine causing depression and visual and auditory hallucinations.20,22 The symptoms in all of these cases resolved with the cessation of clonidine.20,22
Brimonidine has structural and pharmacological similarities to clonidine.8 However, brimonidine penetrates the CNS to a lesser degree because it is less lipophilic.8 This suggests it will result in fewer CNS side effects.8 Both oral and topical brimonidine can cause severe adverse effects such as hypotension, bradycardia, hypotony, hypothermia, and even coma in small children.3,9,22,23 Severe side effects occur in less than 3% of patients using the ophthalmic preparation of brimonidine.24 50-83% of children under the age of 7 experienced somnolence.24 Case reports of severe side effects, including coma, have been reported.24 The increased sensitivity to brimonidine in infants and children could be because of their size (there is no dosage adjustment made based on weight), immature metabolism, immature blood-brain barrier, or increased alpha-2-adrenergic receptor sensitivity.26 As such, brimonidine tartrate ophthalmic solution (0.1%, 0.15%, and 0.2%) is contraindicated in neonates and infants under the age of two.24,25 Elderly patients may also be more susceptible to the neuropsychiatric effects of brimonidine due to a decrease in cerebral alpha-2-adrenoreceptors.8 However, there is no upper age limit to the use of brimonidine tartrate.24,25
Brimonidine tartrate ophthalmic solution is used to treat ocular hypertension and open-angle glaucoma.8,11,27,28 Brimonidine decreases aqueous humor production and increases uveoscleral outflow to lower IOP.28,29 For lipophilic drugs, such as brimonidine tartrate ophthalmic solution, only around 10% of the active drug is absorbed into the eye via the cornea after topical administration.27 It penetrates the cornea and results in a reduction of the IOP within 1 hour.29 Peak effect is achieved within 2 to 3 hours and the trough drug effect occurs approximately 10 to 14 hours after instillation.27 The systemic half-life of the topical solution is about 2 hours.11,24,30 It is metabolized primarily by the liver and excreted by the kidney.11,24,30 However, systemic drug absorption often occurs via the highly vascular nasal mucosa, after drainage through the nasolacrimal duct, bypassing hepatic metabolism and increasing the risk of systemic toxicities.27 The washout period for brimonidine is up to 6 weeks.30
To the best of the author’s knowledge, there are six previously reported cases of brimonidine associated with CBS. The number of published case reports reflects the under-reported cases of CBS and the rarity of its association to brimonidine.
- Tomsak et al. reported four primary open angle glaucoma subjects who experienced visual hallucinations within five days to 2.5 months after starting brimonidine tartrate therapy (Table 1).22The authors found the visual hallucinations resolved after discontinuation of brimonidine tartrate within a time frame of days to four months. The visual hallucinations were attributed to CBS precipitated by brimonidine tartrate eye drops.3,22
- Garcia-Catalan et. al describes a case of an 81-year-old woman with pseudoexfoliation glaucoma and age-related macular degeneration who developed visual hallucinations after one month of using brimonidine. Visual acuity was finger counting at 1 meter OD and 20/400 OS. There was complete resolution of the hallucinations after suspending brimonidine use. The authors attributed the visual hallucinations to CBS precipitated by brimonidine.6
- Santos-Bueso et al. reported a case of a 30-year-old male with Leber’s hereditary optic neuropathy patient who developed visual hallucinations for a month, which coincided with the initiation of treatment using topical brimonidine.9He saw people and faces that stared at him without speaking. The vision was finger counting at 1 meter in each eye. The hallucinations disappeared partially after 72 hours of discontinuing brimonidine and resolved completely after one week. The authors attributed the visual hallucinations to CBS triggered by brimonidine.18
- Kim reported a case of acute psychosis and delirium in a patient receiving brimonidine eye drops in a 68-year-old man with mild open-angle glaucoma. The patient’s wife reported subtle lapses in memory almost on initiation of treatment. He began exhibiting signs of tiredness and depression. He became more and more delusional. Anxiety overcame him and he worried about irrational concerns, ranging from imminent blindness, death, financial ruin, and even concerns about people spying on him. On the ophthalmic exam, the patient was unkempt and quite confused. He was instructed to discontinue the medication. After leaving the clinic, the son saw that the father had a loaded gun, stating that the police were out to get him. The son took his father to a psychiatric hospital, where the psychiatrist discontinued the brimonidine treatment. Within 48 hours, the patient returned to baseline and his paranoid ideation also dissipated. The patient’s vision was 20/20 in each eye.31
- So et al. described a patient with advanced chronic kidney disease who developed encephalopathy due to brimonidine eye drops.Upon hospital admission, review of medications revealed brimonidine tartrate was initiated one month prior at a dosage of one drop twice a day OD. The condition for which brimonidine was initiated was not reported. Visual acuity and visual fields were not reported. The patient’s mental status significantly improved a week after drug cessation.27
When visual hallucinations are precipitated by a medication, as in this case, the diagnosis may be controversial. This is because visual hallucination is often a direct side effect of certain medications. Several medications are associated with visual hallucinations, including those used to treat hypertension, erectile dysfunction, psychiatric and mood disorder.s, movement disorders like Parkinson’s Disease, and some antibiotics.16 In response to Tomsak’s four case reports of CBS precipitated by brimonidine, Rahman argued this point. He questioned whether the visual hallucinations could be a rarer side effect of brimonidine, instead of directly causative for CBS.13 However, brimonidine associated visual hallucinations have not been reported in patients with 20/20 visual acuity and full field of vision. If visual hallucination was a direct side effect of brimonidine, it would not only be limited to patients with visual impairment.
The etiology of the visual hallucinations in the patient presented was presumed to be the brimonidine tartrate 0.2% ophthalmic solution. The visual impairment is consistent with the vision reported in CBS patients, with respect to both the central visual acuity reported by Gilmour et al. and peripheral vision loss reported by Tomsak et. al.7,22 Differentials for his visual hallucinations can include dementia or a side effect of one of his systemic medications. The patient was taking lisinopril and rosuvastatin, both of which are known to cause visual hallucinations.32,33 However, both lisinopril and rosuvastatin were chronic medications the patient began prior to and after the resolution of the visual hallucinations. The patient was not referred for a neurological evaluation because he and his daughter did not report changes to his mood and behavior. The visual hallucinations were not causing the patient stress and he retained insight that the hallucinations were not real. He was able to control the duration of the images by blinking and changing fixation. If neurological or behavioral changes were observed, or if the hallucinations did not resolve with cessation of brimonidine therapy, he would have been referred for a neurological evaluation. Fortunately, the patient continued to be cognitively intact, without signs of dementia, at the one-year retina follow up. The strongest evidence that suggests brimonidine was the offending agent is the temporal relationship of the visual hallucinations and brimonidine therapy. The time of onset and resolution of visual hallucinations was synchronous to the initiation and discontinuation of topical brimonidine. A reasonable association can be made between the visual symptoms and medication. The author proposes that the visual hallucinations are not a side effect of the brimonidine itself, but that brimonidine is a triggering factor to stimulate CBS in at-risk individuals, much like stress and fatigue. To the best of the author’s knowledge, there has not been a report of CBS continuing after the cessation of brimonidine tartrate ophthalmic solution.
In the management of CBS patients, pharmacologic agents have shown mixed efficacy.3 Antipsychotics, anticonvulsants, anti-anxiety, and selective serotonin reuptake inhibitors have been shown, with inconsistent results, to reduce or eliminate visual hallucinations associated with CBS.3 The studies utilized small case reports or case series and provided short-term results.3 Therefore, it is difficult to determine whether the results will transfer to a larger population of patients and can be sustained for a long period of time.3
The patient’s remaining vision should be maximized with spectacles, contact lenses, optical aides, and/or low vision rehabilitation.3 Depending on the etiology of visual impairment, best-corrected spectacles, ground in prism, monocular telescopes, tinted sunglasses, and nightlights may be used.3 By improving the vision, it is possible to reduce the frequency of the visual hallucinations.3 If surgical intervention is appropriate, treating the underlying cause of visual impairment can halt visual hallucinations.3 In a study with 220 patients with neovascular age-related macular degeneration who had treatment with intravitreal ranibizumab injections, 22 of them experienced CBS.3 Five of them reported an improvement in their symptoms (decrease in frequency, decrease in intensity, or complete resolution of visual hallucinations) if their visual acuity improved after the injection.3
One of the most important elements of managing CBS patients is educating the patient and their family about the condition.3 The eye care provider should provide education and reassurance that the condition may be a normal response after vision loss and is not a sign of cognitive decline.3 It is beneficial to inform patients that although visual hallucinations usually resolve spontaneously, they may persist for years in some cases.3 Learning that CBS is unrelated to mental illness relieves concern among patients and their families.3 Eye care practitioners should consider proactively warning low vision patients about the possibility of visual hallucinations after diagnosing their eye condition.3
CONCLUSION
With population life expectancy increasing and the improvement in access to medical care, eye care professionals will likely be seeing more patients with visual impairment.3 As front-line eye care providers, optometrists should be aware of Charles Bonnet Syndrome and recognize the association of brimonidine tartrate ophthalmic solution and visual hallucinations in cognitively intact individuals. Treatment should be provided to optimize the patient’s vision and ocular health, and providers should make appropriate referrals to mental health professionals if the visual hallucinations are causing the patient distress or do not fit the characteristics of Charles Bonnet Syndrome. Eye care providers play a critical role in reassuring patients and their families that the visual hallucinations of CBS do not indicate a cognitive decline. Furthermore, eye care providers are encouraged to rise to the challenge of talking to visually impaired patients about the potential for CBS, especially if initiating brimonidine therapy.
References
-
- Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet Syndrome. Surv Ophthalmol. 2003 Jan-Feb;48(1):58-72.
- Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Visual hallucinations in psychologically normal people: Charles Bonnet’s syndrome. Lancet. 1996 Mar 23;347(9004):794-7.
- Pang L. Hallucinations Experienced by Visually Impaired: Charles Bonnet Syndrome. Optom Vis Sci. 2016 Dec;93(12):1466-1478.
- Shiraishi Y, Terao T, Ibi K, et al. Charles Bonnet syndrome and visual acuity–the involvement of dynamic or acute sensory deprivation. Eur Arch Psychiatry Clin Neurosci. 2004 Dec;254(6):362-4.
- Mansuri Z, Patel K, Shah B, et al. Charles Bonnet Syndrome: A Case Report and Review of the Literature. J Nerv Ment Dis. 2022 Nov 1;210(11):880-882.
- García-Catalán MR, Arriola-Villalobos P, Santos-Bueso E, et al. Síndrome de Charles Bonnet desencadenado por brimonidina [Charles Bonnet syndrome precipitated by brimonidine]. Arch Soc Esp Oftalmol. 2013 Sep;88(9):362-4. Spanish.
- Gilmour G, Schreiber C, Ewing C. An examination of the relationship between low vision and Charles Bonnet syndrome. Can J Ophthalmol. 2009 Feb;44(1):49-52.
- Cimolai N. A review of neuropsychiatric adverse events from topical ophthalmic brimonidine. Hum Exp Toxicol. 2020 Oct;39(10):1279-1290.
- Peters D, Molander S, Lomo T, et al. Charles Bonnet Syndrome in Patients with Open-Angle Glaucoma: Prevalence and Correlation to Visual Field Loss. Ophthalmol Glaucoma. 2022 May-Jun;5(3):337-344.
- Wilkinson F. Auras and other hallucinations: windows on the visual brain. Prog Brain Res. 2004;144:305-20.
- Shagalov DR, Taylor D, Schleichert R, et al. Association of Central Nervous System Depression With Topical Brimonidine When Used for Hemostasis: A Serious Adverse Event. JAMA Dermatol. 2017 Jun 1;153(6):575-577.
- Teunisse R, Zitman F, Raes D. Clinical Evaluation of 14 Patients With the Charles Bonnet Syndrome (Isolated Visual Hallucinations). Comprehensive Psychiatry. 1994 January/February;35(1):70-75.
- Rahman I, Fernando B, Harrison M. Charles Bonnet syndrome and brimonidine: comments. Br J Ophthalmol. 2004 May;88(5):724.
- Lim LW, Li KZ, Tan CS. The diagnosis of Charles Bonnet syndrome in visual field defects. Indian J Endocrinol Metab. 2013 May;17(3):534.
- Kester EM. Charles Bonnet syndrome: case presentation and literature review. Optometry. 2009 Jul;80(7):360-6.
- American Academy of Ophthalmology (AAO). What is Charles Bonnet Syndrome?; 2022. Available at: https:www.aao.org/eye-health/diseases/what-is-charles-bonnet-syndrome. Accessed August 15. 2023.
- Collerton D, Taylor JP. Advances in the treatment of visual hallucinations in neurodegenerative diseases. Future Neurol. 2013 Jul;8(4):433-444.
- Santos-Bueso E, Sáenz-Francés F, Porta-Etessam J, et al. Síndrome de Charles Bonnet desencadenado por brimonidina en paciente con neuropatía óptica hereditaria de Leber . Rev Psiquiatr Salud Ment. 2014 Jul-Sep;7(3):152-3.
- Vacchiano V, Tonon C, Mitolo M, et al. Functional MRI study in a case of Charles Bonnet syndrome related to LHON. BMC Neurol. 2019 Dec 30;19(1):350.
- Brown MJ, Salmon D, Rendell M. Clonidine hallucinations. Ann Intern Med. 1980 Sep;93(3):456-7. doi: 10.7326/0003-4819-93-3-456. PMID: 7436168.
- Delaney J, Spevack D, Doddamani S, et al. Clonidine-induced delirium. Int J Cardiol. 2006 Nov 10;113(2):276-8.
- Tomsak RL, Zaret CR, Weidenthal D. Charles Bonnet syndrome precipitated by brimonidine tartrate eye drops. Br J Ophthalmol. 2003 Jul;87(7):917.
- Berlin RJ, Lee UT, Samples JR, Rich LF, Tang-Liu DD, Sing KA, Steiner RD. Ophthalmic drops causing coma in an infant. J Pediatr. 2001 Mar;138(3):441-3.
- Alphagan [package insert]. Irvine, CA: Allergan; 2016.
- Alphagan P [package insert]. Irvine, CA: Allergan; 2013.
- Bowman, R., Cope, J. & Nischal, K. Ocular and systemic side effects of brimonidine 0.2% eye drops (Alphagan®) in children. Eye 18, 24–26 (2004).
- So BYF, Lee KM, Tang AHC, et al. Brimonidine Eye Drops Causing Encephalopathy in a Patient With Advanced Chronic Kidney Disease. Cureus. 2021 Sep 5;13(9):e17725.
- Gao H, Qiao X, Cantor LB, et al. Up-regulation of brain-derived neurotrophic factor expression by brimonidine in rat retinal ganglion cells. Arch Ophthalmol. 2002 Jun;120(6):797-803.
- Galanopoulos A, Goldberg I. Clinical efficacy and neuroprotective effects of brimonidine in the management of glaucoma and ocular hypertension. Clin Ophthalmol. 2009;3:117-22.
- Stewart WC, Holmes KT, Johnson MA. Washout periods for brimonidine 0.2% and latanoprost 0.005%. Am J Ophthalmol. 2001 Jun;131(6):798-9.
- Kim DD. A case of suspected alphagan-induced psychosis. Arch Ophthalmol. 2000 Aug;118(8):1132-3.
- Doane J, Stults B. Visual hallucinations related to angiotensin-converting enzyme inhibitor use: case reports and review. J Clin Hypertens (Greenwich). 2013 Apr;15(4):230-3.
- Tuccori M, Montagnani S, Mantarro S, Capogrosso-Sansone A, Ruggiero E, Saporiti A, Antonioli L, Fornai M, Blandizzi C. Neuropsychiatric adverse events associated with statins: epidemiology, pathophysiology, prevention and management. CNS Drugs. 2014 Mar;28(3):249-72.
Dr. Kim is the residency coordinator at the Captain James A. Lovell Federal Health Care Center. She is also adjunct faculty at the Illinois College of Optometry.












