Neovascular Glaucoma: A Case Report and Literature Review

Abstract
Background: Neovascular glaucoma is a unique, severe form of glaucoma arising from retinal ischemia. It is often a product of ocular ischemic disorders, such as diabetic retinopathy, ocular ischemic syndrome, and central retinal vein occlusion, all of which may be caused by serious systemic disorders. Neovascular glaucoma must be addressed urgently at onset due to its guarded visual prognosis, the accompanying pain due to the rise in intraocular pressure, and to address the significant underlying systemic etiology.
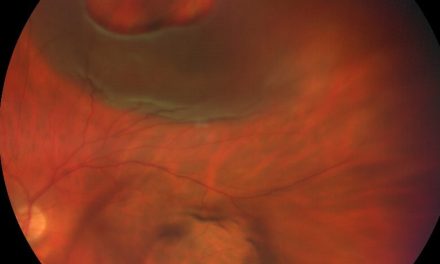
Case Report: A 96-year-old Caucasian male reported for examination as a new patient, transferring care from another provider. His entering visual acuity with correction was 20/30-2 OD and no light perception OS and there was a 4+ relative afferent pupillary defect OS. Goldmann applanation tonometry revealed IOPs of 22mHg OD and 62mmHg OS. The optic nerve for OS had a cup-to-disc ratio of 0.25 round, diffuse pallor, without any collaterals or neovascularization of the disc present. Retinal vessels in OS were attenuated with an artery-to-vein ratio of 1/3 with scattered dot and blot hemorrhages and microaneurysms noted 360 degrees in posterior pole.The peripheral retina OS had scattered areas of panretinal photocoagulation scarring along with mild-moderate dot and blot hemorrhages and microaneurysms 360 degrees.
Conclusion: Neovascular glaucoma is a visually devastating ocular disease arising from serious etiologies, usually proliferative diabetic retinopathy, central retinal vein occlusion, and ocular ischemic syndrome with potentially serious underlying systemic issues. Team management is essential to prevent pain and vision loss in the affected eye, protect the fellow eye and reduce other end-organ damage and mortality.
Keywords: neovascular glaucoma, retinal ischemia, rubeosis, proliferative diabetic retinopathy, central retinal vein occlusion, and ocular ischemic syndrome
Introduction
Neovascular glaucoma is a unique, severe form of glaucoma arising from retinal ischemia. It is often a product of ocular ischemic disorders, such as diabetic retinopathy, ocular ischemic syndrome, and central retinal vein occlusion, all of which may be caused by serious systemic disorders. Neovascular glaucoma must be addressed urgently at onset due to its guarded visual prognosis, the accompanying pain due to the rise in intraocular pressure, and to address the significant underlying systemic etiology. Neovascular glaucoma occurs due to new vasculature obstructing aqueous humor outflow, stemming from posterior segment ischemia.1 Its estimated prevalence is about 3.9% of all glaucoma.2 This disease most often affects the elderly due to cardiovascular risk factors such as hypertension, diabetes, and may be more aggressive in those with obstructive sleep apnea.3 Understanding its underlying etiology is key to prevention and reducing other systemic manifestations. This case will discuss a 96-year-old patient diagnosed with neovascular glaucoma secondary to ocular ischemic syndrome and management directed by evidence-based literature.
Case Report
A 96-year-old Caucasian male reported for examination as a new patient, transferring care from another provider. His ocular history was positive for ocular ischemic syndrome OS, neovascular glaucoma OS, ocular hypertension OD, dry eye disease OU, and severe non-exudative age-related macular degeneration OU. His ocular medications included latanoprost 0.005% qhs OU, dorzolamide 22.3/timolol 6.8 BID OU, brimonidine 0.2% TID OS, artificial tears BID OU, and AREDS2 BID PO. His past ocular surgical history was positive for pseudophakia OU ten years ago, anti-VEGF (Bevacizumab) OS six months ago, and panretinal photocoagulation OS six months ago. His family ocular history was positive for age related macular degeneration (mother). His systemic conditions included hyperlipidemia, benign prostatic hyperplasia, carotid artery narrowing, acute non-ST segment elevation myocardial infarction, malignant tumor of kidney, and chronic kidney disease stage 3. After his diagnosis of ocular ischemic syndrome, he had a carotid doppler performed, which revealed no internal carotid artery stenosis on either side.
His entering visual acuity with correction was 20/30-2 OD and no light perception OS. While the pupils were round and reactive to light, OS was 2.0mm bigger than OD in bright and dim illumination while exhibiting a 4+ relative afferent pupillary defect. Extraocular muscles were normal, confrontation visual fields full OD and OS was not determined as he was unable to fixate on any target.
Slit lamp examination revealed mild lagophthalmos OU and mild ectropion OS. The conjunctiva OD was unremarkable with 3+ diffuse injection OS. The cornea OD was unremarkable but 3+ diffuse punctate epithelial keratitis was present OS. The anterior chamber was deep and quiet OU. The iris was unremarkable OD but showed a small tuft of neovascularization at 5 o’clock OS. Both lenses had a posterior capsular lens clear and centrally in place.
Goldmann applanation tonometry revealed IOPs of 22mHg OD and 62mmHg OS at 2:18 PM.
Gonioscopy was attempted but unfortunately the patient had difficulty tolerating the procedure. Ideally, gonioscopy should be performed on all suspected neovascular glaucoma patients.
He was dilated using tropicamide 1% and phenylephrine 2.5% OD only. Slit lamp biomicroscopy found both eyes to have a posterior vitreous detachment. The optic nerve for OD was judged to have a cup-to-disc ratio of 0.25 round with pink and healthy rims while OS had a cup-to-disc ratio of 0.25 round, diffuse pallor, without any collaterals or neovascularization of the disc present. The right macula displayed a central 1-disc diameter C-shaped geographic atrophy while the left eye displayed central 0.50-disc diameter geographic atrophy. Neither eye exhibited any macular neovascularization. The vessels of OD were judged to be of normal caliber with an artery-to-vein ratio of 2/3. In contrast, the vessels in OS were attenuated with an artery-to-vein ratio of 1/3 with scattered dot and blot hemorrhages and microaneurysms noted 360 degrees in posterior pole. Binocular indirect ophthalmoscope found the OD retina was flat and intact 360 degrees whereas OS had scattered areas of panretinal photocoagulation scarring along with mild-moderate dot and blot hemorrhages and microaneurysms 360 degrees. Given that the patient has no pain and no light perception vision, he was asked to continue his present ocular medication and will continue to be followed closely. He was to call immediately if there was any new ocular pain.
Discussion
Retinal ischemia from the underlying etiology will produce an imbalance between pro-angiogenic and anti-angiogenic factors. This releases vascular endothelial growth factor (VEGF) from the non-pigmented epithelium of the ciliary body, causing new blood vessel formation in the anterior segment of the eye, or neovascularization of the iris (NVI).4 If not properly addressed, transforming growth factors beta 1 and 2 and other angiogenic factors will promote the development of neovascularization within the posterior segment of the eye and cause a fibrovascular membrane to develop over the iris and within the anterior chamber angle, referred to neovascularization of the angle (NVA).5
Neovascular glaucoma (NVG) can be divided into four stages, the pre-rubeosis stage, preglaucoma stage, open angle stage, and closed angle stage.1 The pre-rubeosis stage is defined as the absence of symptoms, normal IOP, though clinical findings, such as hemorrhaging in central retinal vein occlusion, may be present. This is considered a precursor to NVG and where intervention is most effective. The preglaucoma stage is defined as lack of symptoms, normal IOP and presence of NVI. Neovascularization of the iris is best identified by carefully examining the iris for any fine, aberrant vessels at the pupillary border pre-dilation under high magnification, i.e., 25x, 40x. NVI will appear more tortuous and have a more vivid hue compared to iris blood vessels.6 Before it advances into the angle, the IOP may be normal, especially if the patient does not have any pre-existing glaucoma.7 This is the stage where gonioscopy will aid clinicians by showing NVA and any peripheral anterior synechiae present. Peripheral anterior synechiae occur when the iris adheres to the angle. In the case of NVG, this begins to develop due to the formation of fibrovascular membranes, which contributes to elevated IOP and angle closure. The open angle glaucoma stage has NVI and NVA present, and fibrovascular membranes forming in the trabecular meshwork causing rise in the IOP. NVA will show new vessels extending beyond the ciliary body band and scleral spur onto the surface of the trabecular meshwork, whereas normal vessels do not typically go past the scleral spur.8 The final stage, closed angle stage, is where patients are quite symptomatic due to elevated IOP. Clinically, patients will display a fixated pupil, corneal edema, anterior segment inflammation such as anterior chamber cells and flare, ocular pain, profound vision loss, ectropian uveae, and elevated IOP, often exceeding 60mmHg.1,9 Vision loss can range from 20/40 to no light perception with 95% of eyes becoming counting fingers or worse within one year.10,11 Hyphema may occur due to leakage from the new blood vessels.12 Patients with NVG may be asymptomatic in the early stages of the disease, if the IOP rise is gradual, and if the corneal endothelial count is good.1
The three most common causes of NVG are diabetic retinopathy, central retinal vein occlusion (CRVO), and ocular ischemic syndrome (OIS).13 It is estimated about one-third of NVG cases are due to proliferative diabetic retinopathy. Approximately 65% of these eyes develop NVI, 5-8% develop NVG, and 33% develop NVG in the contralateral eye if NVG is already present in one eye.14,15 In untreated cases of diabetic retinopathy, the amount of time between the onset of NVI and development of NVG is typically one month to three years.16 NVG is strongly associated with duration of diabetic disease and poor glycemic control. NVG is rarely associated with non-proliferative diabetic retinopathy unless there is carotid occlusive disease present. CRVO contributes to another one-third of NVG cases and tends to be more aggressive compared to those of diabetic origin.17 NVG can occur within a few weeks to two years after a diagnosis of CRVO. Indeed, NVG from CRVO is often referred to as “90-day glaucoma” due to its 3-month latency period.19 Eyes with non-ischemic CRVO have a 3.3% risk of conversion to an ischemic CRVO within four months and ten times higher incidence within three years. This risk is increased if there is associated diabetic retinopathy or OIS.20 The rate of incidence of NVG is about 40-45% in ischemic CRVO cases and 10% in nonischemic CRVO cases.14 Eyes with larger areas of capillary nonperfusion, 30-75 disc areas, are at a higher risk of developing NVG.21 OIS contributes to about 13% of NVG cases due to decreased perfusion to the eye and/or reversal of blood flow in the ophthalmic artery caused by either stenosis/occlusion of the common or internal carotid arteries or reduced collateral circulation between the internal and external carotid arteries.14,22,23 Other common causes of OIS include atherosclerosis, myocardial infarction, and diabetes mellitus.11
Less common etiologies of NVG may include central retinal artery occlusion, branch retinal artery occlusion, branch retinal vein occlusion, giant cell arteritis Takayasu arteritis, retinal detachment, retinopathy of prematurity, sickle cell retinopathy, Eales disease, Coat’s disease, Leber’s congenital amaurosis, retinoschisis, persistent hyperplastic primary vitreous, syphilitic posterior uveitis and vasculitis, and optic nerve glioma with venous stasis retinopathy.24
Management of NVG requires treating the underlying pathology. In diabetic patients, blood glucose levels and a glycated hemoglobin (HbA1c) should be obtained if unknown and systemic treatment should be discussed with patient along with their primary care physician and/or endocrinologist. CRVO cases should be comanaged with primary care to address potential vascular disease of multiple etiologies.25 For OIS patients, it is necessary to obtain a carotid doppler of the internal carotid arteries to assess for stenosis/occlusion as more than 90% of patients with OIS have ipsilateral carotid stenosis.26 A complete occlusion may found on the affected side in 50% of these patients.27 The use of anti-platelet and anti-coagulant medication should be discussed along with team management involving primary care, cardiology, and/or a vascular surgeon, especially as OIS carries a mortality rate as high as 40% within 5 years at the time of diagnosis.21,28,23
Panretinal photocoagulation (PRP) is the first line treatment of NVG by reducing retinal ischemia thus reducing the amount of anti-VEGF being released and limiting neovascularization within the eye.29-32 In PRP, a spot size of 500µm-800µm and up to 2,000 laser burns are applied to the outer retina completed in 1-3 sessions over 5-7 days. PRP causes not only regression but involution of anterior segment neovascularization in 60% of cases. Treatment also helps reduce the IOP and induces vessel regression throughout the posterior segment.33 However, in cases of uncontrolled, high IOP, administration of PRP can sometimes increase the IOP due to cilio-choroidal effusion. In these cases, it is necessary to either control the IOP first or administer anti-VEGF.34 In OIS cases, PRP alone is often not sufficient and further intervention is necessary.9
Anti-VEGF therapy such as bevacizumab, ranibizumab and aflibercept have all clinically proven to be sufficient to help regress neovascularization in as little as one day after an injection.35-40 Anti-VEGF therapy is currently used as an adjunct to PRP.41,42
Quick and aggressive treatment is necessary to control the IOP, inflammation, and ischemia present. Upon initial presentation, a cycloplegic agent such as atropine 1% BID in addition to a steroid such as prednisolone acetate 1% or difluprednate 0.05% QID should be prescribed.24 Even if the angle is closed due to peripheral anterior synechiae, atropine is still warranted. Topical steroids may seem counterintuitive as they can raise the IOP further, however, they will increase patient comfort and decrease inflammation. Aqueous suppressants such as beta blockers, carbonic anhydrase inhibitors, and alpha-adrenergic agonists may be initially used to decrease the IOP.12 Miotics such as pilocarpine are contraindicated to use as they are pro-inflammatory. Prostaglandin analogs may exacerbate inflammation in the acute phase of NVG but are effective once the IOP is uncontrolled by other medical means.
Trabeculectomy has limited success in NVG cases. Intraoperative bleeding caused by fibrovascular membranes is a common complication. Use of metabolites such as 5-flourouracil, mitomycin C and prior surgical treatment such as PRP to regress the amount of NVI and NVA present may improve outcomes, however, its success rate is about 51.3% at 5 years.44 Tube shunts also have a low success rate of 62% at one year but only 10% at five years with CRVO having a worse prognosis compared to diabetic retinopathy.45 Cyclophotocoagulation uses a diode laser to decrease aqueous production and reduce IOP. It is reserved for eyes with poor visual prognosis or to reduce the high IOP when the media is not clear enough to perform a PRP/other filtering surgeries are not feasible.46 Lastly, a retrobulbar alcohol injection may be considered if medical and surgical treatments fail to manage the pain. Ultimately, if the pain cannot be controlled, patients may need to undergo enucleation.47 For NVG eyes that have a visual acuity of no light perception and lack of pain, despite high IOP readings, as long as the eye does not have any pain, the patient can be periodically observed.48
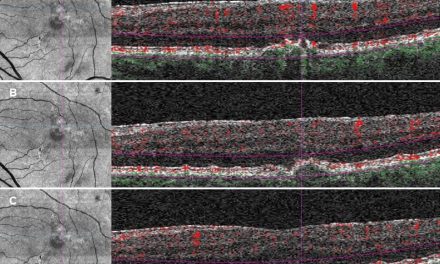
Fluorescein angiography and optical coherence tomography angiography (OCTA) are two other management tools practitioners may use to further evaluate patients with NVG. Fluorescein angiography assesses capillary nonperfusion, retinal ischemia, and iris neovascularization whereas OCTA has the capability of detecting the extent and depth of neovascularization and its regression upon follow-up.49,50
The prognosis with NVG is guarded and depends on prevention and treatment of NVG early in its course along with treating the underlying disease process. NVG is associated with a high mortality rate.1 The goal of treatment is to reduce ocular pain, prevent further vision loss and prevent NVG from occurring in the fellow eye by identifying the underlying etiology. This goal is critical in helping reduce systemic sequelae, such as mortality. Prevention is key in patients who are diagnosed with diabetes mellitus, arteriosclerosis, atherosclerosis, and hypercoagulability states. These patients should be monitored very closely and risk factors/preventative measures should be discussed with them.
Conclusion
Neovascular glaucoma is a visually devastating ocular disease arising from serious etiologies, usually proliferative diabetic retinopathy, central retinal vein occlusion, and ocular ischemic syndrome with potentially serious underlying systemic issues. Team management is essential to prevent pain and vision loss in the affected eye, protect the fellow eye and reduce other end-organ damage and mortality. Our patient demonstrated end-stage neovascular glaucoma secondary to ocular ischemic syndrome despite therapeutic intervention. Going forward for this patient, the goals are comfort OS and preserving vision OD. Co-management with other specialties including primary care, ophthalmology, and cardiology are necessary to not only help obtain these goals but reduce systemic risks overall.
References
- Shazly TA, Latina MA. Neovascular glaucoma: etiology, diagnosis and prognosis. Semin Ophthalmol. 2009;24(2):113–21.
- Shiba T, Sato Y, Takahashi M. Relationship between diabetic retinopathy and sleep-disordered breathing. Am J Ophthalmol. 2009 Jun;147(6):1017-21.
- Shiba T, Takahashi M, Hori Y, Saishin Y, Sato Y, Maeno T. Relationship between sleep-disordered breathing and iris and/or angle neovascularization in proliferative diabetic retinopathy cases. Am J Ophthalmol. 2011 Apr;151(4):604-9.
- Chalam KV, Brar VS, Murthy RK. Human ciliary epithelium as a source of synthesis and secretion of vascular endothelial growth factor in neovascular glaucoma. JAMA Ophthalmol. 2014 Nov;132(11):1350-4.
- Tripathi RC, Borisuth NS, Tripathi BJ. Detection, quantification, and significance of basic fibroblast growth factor in the aqueous humor of man, cat, dog and pig. Exp Eye Res. 1992 Mar;54(3):447-54.
- Chen HF, Chen MC, Lai CC, Yeung L, Wang NK, Chen HSL, Ku WC, Wu SC, Chang SHL, Chuang LH. Neovascular glaucoma after central retinal vein occlusion in pre-existing glaucoma. BMC Ophthalmol. 2014;14:119.
- Kumawat D, Sahay P, Shah P. Comment on: predictors of neovascular glaucoma in central retinal vein occlusion. Am J Ophthalmol. 2020;209:217–218.
- Călugăru D, Călugăru M. Comment on: ‘Long-term outcomes of neovascular glaucoma treated with and without intravitreal bevacizumab’ Eye (Lond) 2016;30(6):896–897.
- Löffler KU. Neovascular glaucoma: aetiology, pathogenesis and treatment. Ophthalmolog 2006;103(12):1057-63.
- Kuang T, Ling Liu C, Chou C, Hsu W. Clinical experience in the management of neovascular glaucoma. J Chin Med Assoc. 2004 Mar;67(3):131-5.
- Sivalingam A, Brown GC, Magargal LE. The ocular ischaemic syndrome. Visual prognosis and the effect of treatment. Int Ophthalmol. 1991; 15:15–20.
- Nakatake S, Yoshida S, Nakao S, Arita R, Yasuda M, Kita T, Enaida H, Ohshima Y, Ishibashi T. Hyphema is a risk factor for failure of trabeculectomy in neovascular glaucoma: a retrospective analysis. BMC Ophthalmol. 2014;14:55.
- Senthil S, Dada T, Das T, Kaushik S, Puthuran GV, Philip R, Rani PK, Ra to be over there however do not get 3 sets o H, Singla S, Vijaya L. Neovascular glaucoma – A review. Indian J Ophthalmol. 2021 Mar;69(3):525-534.
- Widder R, Lemmen KD, Dietlein T. Neovaskularisationsglaukom. Klin Monatsbl Augenheilkd. 2010;227(2):R15–R28.
- Lee P, Wang CC, Adamis AP. Ocular neovascularization: An epidemiologic review. Surv Ophthalmol. 1998;43:245–69.
- Madsen PH. Rubeosis of the iris and haemorrhagic glaucoma in patients with proliferative diabetic retinopathy. Br J Ophthalmol. 1971;55:368-71.
- Scruggs B, Quist T, Syed N, Alward W. Neovascular glaucoma.
- Lim LL, Cheung N, Wang JJ, Islam FM, Mitchell P, Saw SM, Aung T, Wong TY. Prevalence and risk factors of retinal vein occlusion in an Asian population. Br J Ophthalmol. 2008 Oct;92(10):1316-9.
- Mocanu C, Barăscu D, Marinescu F, Lăcrăţeanu M, Iliuşi F, Simionescu C. [Neovascular glaucoma–retrospective study]. Oftalmologia. 2005;49(4):58-65.
- Baseline and early natural history report. The Central Vein Occlusion Study. Arch 1993 Aug;111(8):1087-95.
- Hayreh SS. The CVOS group M and N reports. 1996;103:350–2.
- Rong AJ, Swaminathan SS, Vanner EA, Parrish RK., 2nd Predictors of neovascular glaucoma in central retinal vein occlusion. Am J Ophthalmol. 2019;204:62–69.
- Mendrinos E, Machinie TG, Pournaras CJ. Ocular Ischemic Syndrome. Surv Ophthalmol. 2010;55(1):2–34.
- Sivak-Callcott JA, O’Day DM, Gass JD, Tsai JC. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. 2001 Oct;108(10):1767-76.
- Hayreh SS, Zimmerman MS, Podhajsky PA. Hematologic abnormalities associated with various types of retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2002;240:180-96.
- Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome: a systematic review. Med Sci Monit. 2012; 18:RA138–RA144.
- Magargal LE, Sanborn GE, Zimmerman A. Venous stasis retinopathy associated with embolic obstruction of the central retinal artery. J Clin Neuroophthalmol.1982; 2:113–118.
- Kumar, S, Kumari, S, Sinha AK, Kumar S, Kumari S, Sinha Neovascular Glaucoma: An Update on Etiopathogenesis, Diagnostics and Management. Open J. Ophthalmol. 2022;12:242–258.
- Brooks AM, Gillies WE.The development and management of neovascular glaucoma. Aust N Z J Ophthalmol. 1990;18(2):179-85.
- Cashwell LF, Marks WP. Panretinal photocoagulation in the management of neovascular glaucoma. South Med J. 1988;81(11):1364-8.
- Stefaniotou M, Paschides CA, Psilas K. Panretinal cryopexy for the management of neovascularization of the iris. Ophthalmologica. 1995;209(3):141-4.
- Lee SC, Kim GO, Kim DH, et al. Endoscopic laser photocoagulation for management of neovascular glaucoma. Yonsei Med J. 2000;41(4):445-9.
- Hamard P, Baudouin C. Consensus on neovascular glaucoma. J Fr Ophtalmol. 2000;23(3):289-94.
- Alkawas AA< Shahien EA, Hussein AM. Management of neovascular glaucoma with panretinal photocoagulation, intravitreal bevacizumab, and subsequent trabeculectomy with mitomycin C. J Glaucoma. 2010;19:622-6.
- Lee SJ, Lee JJ, Kim SY, Kim SD. Intravitreal bevacizumab (Avastin) treatment of neovascular glaucoma in ocular ischemic syndrome. Korean J Ophthalmol. 2009;23(2):132-4.
- Yazdani S, Hendi K, Pakravan M, et al. Intravitreal bevacizumab for neovascular glaucoma: a randomized controlled trial. J Glaucoma. 2009;18(8):632-7.
- Beutel J, Peters S, Lüke M, et al. Bevacizumab as adjuvant for neovascular glaucoma. Acta Ophthalmol. 2010;88(1):103-9.
- Batioğlu F, Astam N, Ozmert E. Rapid improvement of retinal and iris neovascularization after a single intravitreal bevacizumab injection in a patient with central retinal vein occlusion and neovascular glaucoma. Int Ophthalmol. 2008;28(1):59-61.
- Katsanos A, Gorgoli K, Mikropoulos DG, et al Assessing the role of ranibizumab in improving the outcome of glaucoma filtering surgery and neovascular glaucoma. Expert Opin Biol Ther. 2018;18(6):719-24.
- Andrés-Guerrero V, Perucho-González L, García-Feijoo J, et al. Current perspectives on the use of anti-VEGF drugs as adjuvant therapy in glaucoma. Adv Ther. 2017;34(2):378-95.
- Hasanreisoglu M, Weinberger D, Mimouni K, et al. Intravitreal bevacizumab as an adjunct treatment for neovascular glaucoma. Eur J Ophthalmol. 2009;19(4):607-12.
- Ciftci S, Sakalar YB, Unlu K, et al. Intravitreal bevacizumab combined with panretinal photocoagulation in the treatment of open angle neovascular glaucoma. Eur J Ophthalmol. 2009;19(6):1028-33.
- Hayreh SS. Neovascular glaucoma. Prog Retin Eye Res. 2007;26:470–85.
- Takihara Y, Inatani M, Fukushima M, Iwao K, Iwao M, Tanihara H. Trabeculectomy with mitomycin C for neovascular glaucoma: Prognostic factors for surgical failure. Am J Ophthalmol. 2009;147:912–918. e911.
- Mermoud, A. et al. Ophthalmology1993; 100:897–902.
- Bloom PA, Tsai JC, Sharma K, Miller MH, Rice NS, Hitchings RA, et al. “Cyclodiode”: Trans-scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. 1997;104:1508–20.
- Havens SJ, Gulati V. Dev Ophthalmol. 2016;55:196-204.
- Mishra C. Neovascular Glaucoma. 7 Nov. 2022.
- Li ZQ, Zhou XX, Lin S, Li JL, Wu JG. Angiography reveals early hiding iris neovascularization after ischemic CRVO. Int J Ophthalmol. 2013;6:253–254.
- Hwang TS, Hagag AM, Wang J, Zhang M, Smith A, Wilson DJ, et al. Automated quantification of nonperfusion areas in 3 vascular plexuses with optical coherence tomography angiography in eyes of patients with diabetes. JAMA Ophthalmol. 2018;136:929–6.
Dr. Gottehrer completed her Ocular Disease residency in 2017 and her fellowship from American Academy of Optometry in 2018. She currently practices at Veteran Affairs Finger Lakes Healthcare System and enjoys sharing teaching case reports with her peers. Hobbies include spending time with her pets and traveling.