Uveitis: Laboratory Investigations and Current Recommendations on Diagnosis

Abstract
This report describes a case of bilateral anterior granulomatous uveitis in a 52-year-old African American male. He was initially treated and responded to topical steroids (Prednisolone 1% q2h), only to rebound a month later. This prompted an investigation, including laboratory testing, chest x-ray, and rheumatology referral. Ultimately, he was diagnosed with pulmonary sarcoidosis and treated with a combination of topical ophthalmic corticosteroids and systemic immunosuppressants to obtain remission.
Uveitis, especially anterior uveitis, is treated frequently in primary care optometric practices. Managing ocular inflammation is generally straightforward with a combination of topical corticosteroids and mydriatic agents. However, there is no standard diagnostic approach to evaluating the underlying etiology, and often these cases are deemed idiopathic. Case history and clinical examination are imperative when determining which cases are benign versus those with underlying systemic origin, where further clinical assessment should be performed. The anatomical classification of uveitis will be detailed, as well as the most common laboratory tests used to aid in diagnosis of systemic disease.
Case Report
A 52-year-old African American male presented to clinic with complaints of occasional mild ocular redness, blurred vision, and photophobia. He reported his symptoms began 2-3 months prior only to resolve and recur every few days to a week. He stated the redness appeared in both eyes but often alternated. He was overall unbothered by his mild symptoms and elaborated only after inquiry about his slight conjunctival injection.
His medical history was significant for hypertension, anemia, hypercholesterolemia, decreased vitamin D, contracture of the hand joint, and seizure disorder. His medications included atorvastatin, lisinopril, topiramate and a multivitamin. He was a current tobacco user and consumed a minimal amount of alcohol. His family history was noncontributory. He had no prior history of ocular complications. He was unaware of when his last eye exam occurred, but it had been several years prior. During his review of systems, he mentioned he had abdominal lumps that appeared underneath his skin but resolved over a few weeks. He denied any joint pain, back pain, or mouth ulcers.
His entering uncorrected visual acuity measured 20/20- each eye at distance and 20/30 at near. Refraction yielded low hyperopia and presbyopia with correction to 20/20 distance and near in each eye. His pupils were equal, round, and reactive to light. There was no afferent pupillary defect. Confrontation visual fields were normal. His intraocular pressure was obtained with a rebound tonometer, measuring 15mmHg OD and 16mmHg OS.
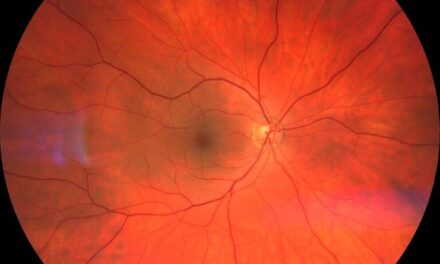
Anterior segment examination revealed mild bilateral limbal conjunctival injection. The right eye had 10-15 large keratic precipitates (KPs) of the corneal endothelium and mild anterior chamber (AC) reaction. The left eye had 15+ KPs and a moderate AC reaction. There was a Koeppe nodule on the inferior nasal margin of his left iris. Iridocorneal angles were open based on Von Herrick’s estimation. His pupils were dilated with 2.5% phenylephrine and 1% tropicamide. The posterior segment examination was unremarkable. No vitritis, retinitis, or vasculitis was observed. His optic nerves were pink and well-perfused.
Based on his 2–3-month history and presenting symptoms, he was diagnosed with chronic bilateral granulomatous anterior uveitis. He was prescribed prednisolone acetate 1% q2 hours in both eyes and was instructed to follow up in 1 week. Lab work was initiated due to the chronicity of his symptoms and granulomatous appearance. The lab tests ordered included antinuclear antibodies (ANA), Lyme antibodies, angiotensin-converting enzyme (ACE), treponemal antibody, HLA B27, C-Reactive protein (CRP), rheumatoid factor (RF), and complete blood count (CBC). Table 1 indicates the results of the initial testing.
Blood testing was unremarkable for any signs of active systemic inflammatory markers. His complete blood count was relatively normal except for mild lymphocytopenia.
There was a several-week delay in follow up, and he returned to the clinic after a month. There was a significant improvement in both clinical and subjective signs and symptoms. He reported relief of redness and improvement in photopsia. His entering visual acuities measured 20/20 in each eye. His intraocular pressure measured 11mmHg OD and 12mmHg OS. In the right eye keratic precipitates were still present, but improved, and there was no anterior chamber reaction. The left eye also had a decrease in the number of keratic precipitates and a trace anterior chamber reaction. The Koeppe nodule was still present in the left eye. He was to continue the prednisolone acetate four times a day and follow up in two weeks to begin tapering.
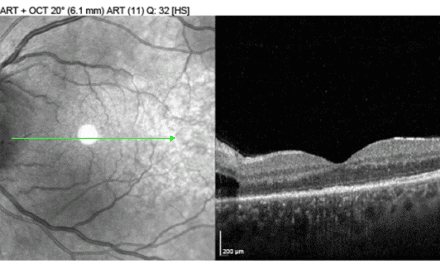
He returned to the clinic two months later, having stopped the prednisone the month prior. He stated the vision in his right eye had become foggy over the last few weeks, but his symptoms were not as severe as his initial presentation. His unaided visual acuities measured 20/20 OD and OS. Tonometry measured 11mmHg OD and 09mmHg OS. He was dilated with 2.5% phenylephrine and 1% tropicamide. Posterior segment examination was normal, except bilateral mild vitreous opacities were now noted in the inferior quadrant.
He was prescribed difluprednate 0.05% ophthalmic emulsion four times a day in both eyes. He was counseled on strict compliance with medication and follow-up schedule. He was also referred to rheumatology at this examination due to posterior segment findings. A recommendation was made to order a chest x-ray and further laboratory workup for chronic recurrent granulomatous uveitis. Sarcoidosis was the preliminary diagnosis due to the bilateral granulomatous uveitis, the “string of pearls” vitreous opacities, and slight lymphocytopenia.
During the 2-week follow-up, his symptoms again improved. His visual acuity measured 20/20 OD, OS, and his tonometry readings measured 21mmHg OD and 19mmHg OS. His examination revealed improved anterior segment findings of the right eye. Approximately 4-5 fine KPs remained, as well as trace cell reaction and no posterior segment findings. The KPs of the left eye had resolved, but the Koeppe nodule remained on the iris. He reported he had an upcoming rheumatology appointment in one week. He was instructed to continue the difluprednate four times a day for two weeks, then plan on a slow taper. He was to follow up in two weeks to begin the taper.
His next follow-up was two months later. He had reduced the difluprednate to two times a day in both eyes. His visual acuity remained unchanged, and his intraocular pressure measured 12mmHg OD and 10mmHg OS. The anterior and posterior segment findings were resolved, and the Koeppe nodule of the left eye was no longer present. He was instructed to continue slow taper over the next month. He had also been examined by rheumatology by this visit and was additionally prescribed methotrexate 15mg weekly and folic acid 1mg daily.
His rheumatology work-up consisted of a chest X-ray and labs evaluating for tuberculosis (TB), hepatitis B, vitamin D, cyclic citrullinated peptide (CCP), comprehensive metabolic panel and hepatitis panel. Their main differentials included sarcoidosis and Bechet’s disease. His blood labs were normal, but his chest X-ray indicated mediastinal and bilateral hilar adenopathy. He subsequently underwent bronchoscopy with a biopsy of lung nodules to rule out malignancy. The results of the rheumatology and pulmonary testing were consistent with stage II sarcoidosis. He is treated for his sarcoid and is symptom-free from ocular and respiratory problems at this time.
Discussion
Uveitis is defined by inflammation of the iris, ciliary body, choroid, retina or vitreous.1 It is the third leading cause of blindness worldwide and encompasses approximately 10% of preventable vision loss in the United States.2 The prevalence of uveitis in the US is around 38 cases per 100,000 and has an incidence of 15 cases per 100,000. However, the epidemiology can differ considerably depending on geographic location, race, age, and genetics.3,4 Untreated uveitis can be sight-threatening due to secondary complications such as macular edema, glaucoma and retinal ischemia.1
Uveitis can be categorized anatomically, etiologically, histologically or based on clinical course.3 Different classification terms and definitions can be confusing when comparing clinical data across research studies, so attempts at standardization have been made. In 2005, the Standardization of Uveitis Nomenclature (SUN) working group met to simplify the classification of uveitis and to standardize the grading of clinical signs. They decided an anatomical classification scheme should be the primary means of categorization and then be further subdivided if necessary.5 Therefore, the four main anatomical categories of uveitis are anterior uveitis, intermediate uveitis, posterior uveitis and panuveitis. See Table 2 for the SUN classification table describing the anatomical sites of each. Each category can be further subdivided based on the clinical course (acute, chronic), histologically (granulomatous or non-granulomatous), or etiology (infectious or non-infectious).4
Anterior uveitis is defined by inflammation originating in the iris and ciliary body and presents as cells and flare observed upon examination of the anterior chamber. It accounts for approximately 65-90% of cases encountered in a primary eye care setting.8 Intermediate uveitis is the least common and occurs in the anterior vitreous and pars plana. Posterior uveitis is the second most common and involves the choroid and retina. When multiple sites are notably involved, the term panuveitis is used.4
Uveitis can pose a clinical diagnostic challenge due to the extensive array of underlying causes. There have been approximately sixty systemic, infectious or ocular syndromes that have been identified.1 Even in tertiary clinical settings, there is a high percentage of cases, 30-40%, in which no underlying cause was found. 2 A thorough case history and comprehensive ophthalmological examination is an important first step in developing a working diagnosis. A patient’s history should consist of their demographics, past medical history, past surgical history, family history, systemic history and drug history.4 The eye care provider is often tasked with undertaking further investigations and comanaging with specialists, such as rheumatology, when history alone fails to point to a diagnosis.
Currently, there is no standard approach for laboratory investigations in patients with uveitis. There are differing opinions on which baseline testing is most valuable and what follow-up testing yields the highest diagnostic rates. A 2017 French study titled ULISSE (Uveitis: cLInical and medicoeconomic evaluation of a Standardized Strategy of the Etiological diagnosis) looked at two different testing strategies. The first was a standardized stepwise testing strategy, in which each patient had the same battery of tests completed in a specific order based on clinical signs and anatomical classification. The next was an open strategy where any test could be performed at any point in the evaluation of the patient. Both strategies were similarly effective and were able to determine the underlying diagnosis roughly half of the time during a period of 6 months.2 However, the ULISSE study did not evaluate the cost-effectiveness of each strategy. An open strategy would likely have a higher financial impact on the patient due to the greater number of tests performed during the investigation.2
When determining which tests will yield the highest rate of return, it is helpful to understand which underlying conditions are most common in the specific category of uveitis and geographic location. For instance, in the United States, toxoplasmosis occurs in a large percentage of patients with posterior uveitis, specifically focal chorioretinitis. But it occurs less often in Asian countries. The belief is Americans have a higher rate of exposure to the parasite Toxoplasma gondii due to a higher percentage of the population that owns cats as pets.8 However, infectious posterior uveitis is encountered much less frequently in the US in comparison to idiopathic anterior uveitis.3 See Table 3 below for the most common causes of uveitis by anatomical type in the United States.
In 2017, Séve et al. published a literature review of current recommendations describing the diagnostic work-up of a uveitis patient. First, they concluded diagnosis should be heavily weighed on the medical history and clinical examination of the patient. Second, syphilis screening should be performed in all cases. Third, further diagnostic testing should be based on the specific anatomical classification.1 Their recommendations are illustrated below in Table 4. They recommended minimal first-line tests for all types of uveitis should include complete blood count (CBC), erythrocyte sedimentation rate (ESR), c-reactive protein (CRP), Tuberculin skin test, Syphilis serology, and chest x-ray. They acknowledge the first-line tests have a low diagnostic rate but are simple to perform. In addition to these tests, HLA-B27 should be performed on patients with acute non-granulomatous anterior uveitis. HSV, VZV and CMV serology are recommended for acute anterior granulomatous uveitis that is unexplained and not responding well to treatment. ACE, chest CT scan, and IGRA are recommended for chronic uveitis to evaluate for sarcoidosis and tuberculosis. A brain MRI should be performed in patients with intermediate uveitis due to the higher prevalence of multiple sclerosis in this type.8 If there is chronic posterior uveitis, specifically focal retinitis, toxoplasma serology is recommended. Lumbar puncture and bronchoalveolar lavage can also be performed as adjunctive investigations if warranted. Antinuclear antibody testing is only useful in a young patient suspected of having juvenile idiopathic arthritis or in adults with peripheral polyarthritis.1
A McKay et al. article titled “Rational laboratory testing in uveitis: A Bayesian analysis” discusses the predictive value of each test depends on the sensitivity and specificity of a specific condition. Their recommendations were similar to P. Séve with slight variations. They recommend CBC with differential, CMP, and syphilis testing as baseline testing for all types of uveitis. He also acknowledges the low probability of diagnosis with this initial battery. They recommend Hepatitis B/C, tuberculosis, and HIV screening for all high-risk patients before initiating immunotherapy treatment. He suggests TB testing should only be performed for endemic areas unless symptoms warrant testing. HLA-B27 testing should only be performed when physical signs warrant testing. They do not recommend HLA-B51 testing due to the low specificity and sensitivity for diagnosing Behcet’s disease. They prefer chest X-ray over ACE and lysozyme testing and state although CT testing is superior to X-ray, it does not improve diagnostic capability. MRI is only recommended in intermediate uveitis when other neurological signs point to multiple sclerosis. They believe anti-nuclear antibodies (ANA), rheumatoid factor (RF), anti-citrullinated protein antibody (anti-CCP) and antineutrophil cytoplasmic antibodies (AMCA) are not useful unless other systemic symptoms are present. The only instance where baseline ANA testing would be appropriate is for a uveitis case presenting in a child under 16.12
Below is a summary of the most common laboratory tests used in uveitis evaluation.
Complete Blood Count (CBC)
Complete blood cell count measures red blood cells, white blood cells, platelets, hemoglobin, hematocrit, and mean corpuscular volume (MCV). In addition, complete blood cell count with differential quantifies the amount of specific white blood cells.13 This test is not a high-yield test for diagnosing uveitis, but it will give general information on the overall health of the patient. Occasionally it can point to differential diagnoses, such as eosinophilia or lymphocytopenia in sarcoidosis. It can also help determine baseline levels before beginning immunosuppressive therapy.13,14
Comprehensive Metabolic Panel (CMP)
CMP measures 14 different blood components and gives information on a person’s metabolism and chemical balance. It measures liver function by measuring albumin, a protein made by the liver, and the liver enzymes, alkaline phosphatase, alanine transaminase and aspartate aminotransferase. It also measures bilirubin, which is a byproduct of the liver. It quantifies kidney function by measuring blood urea nitrogen and creatinine, both waste products of the kidneys. Sodium, potassium, carbon dioxide and chloride are the electrolytes measured. Finally, it measures blood glucose and blood calcium, a vital mineral for nerve, heart, and muscle function. CMP can also aid in the diagnosis of sarcoidosis. Elevated liver enzymes and calcium can point to liver granulomas.15,16
Syphilis serology
Syphilis is caused by the spirochete Treponema pallidum and is known as the great mimicker due to the vast array of presenting symptoms. There has been an upsurge in prevalence in recent years, and it can present with all forms of uveitis, mainly panuveitis. 17 Syphilis is an important condition to exclude because of its non-specific symptoms and increased morbidity if left undiagnosed. Therefore it is recommended to screen all patients who present with uveitis.1,17 Syphilis diagnostics can be complex and there are an array of various testing strategies. According to the Centers for Disease Control, “A presumptive diagnosis of syphilis requires the use of two laboratory serologic tests: a nontreponemal test (i.e., Venereal Disease Research Laboratory [VDRL] or rapid plasma reagin [RPR] test) and a treponemal test (i.e., the T. pallidum passive particle agglutination [TP-PA] assay, various EIAs, chemiluminescence immunoassays [CIAs] and immunoblots, or rapid treponemal assays).”18
Radiologic testing
Chest X-ray and thoracic computed tomography (CT) are used to examine tuberculosis and sarcoidosis. Sarcoidosis is suspected if bilateral hilar or mediastinal adenopathy is observed. Pulmonary tuberculosis can cause “scarring of the lung apex, pleural/parenchymatous calcifications, or unilateral calcified hilar adenopathy”.1 Thoracic CT scans have a greater sensitivity and specificity to both conditions. However, it has a greater financial cost and exposes the patient to radiation 12-450 times of that of a chest x-ray.12
HLA -B27
Human leukocyte antigens (HLA) are found on the surface of all cells and help the immune system differentiate self from non-self. The specific antigen HLA-B27 is found in 3-6% of the Caucasian population but is found in up to 90% of patients who have seronegative spondyloarthropathies (rheumatoid factor and antinuclear antibody negative). These conditions include ankylosing spondylitis, reactive arthritis, psoriatic arthritis, Crohn’s disease, and ulcerative arthritis. This test is completed with a small sample of whole blood obtained by venous draw, and it is evaluated by polymerase chain reaction.19,20
HLA-B51
Behcet’s syndrome is a multisystem inflammatory condition consisting of recurring oral and genital ulcers, uveitis, and arthritis. It also can involve the gastrointestinal and central nervous systems. There is a higher prevalence in the Mediterranean basin, Middle Eastern and Far East Asian Countries, often known as the “Silk Trade Route.” 50-80% of patients with Behcet’s syndrome test positive for this antigen. The United States has a much lower rate of Behcet’s disease than these countries, even within populations who have immigrated from these countries; therefore, there appear also to be environmental factors that play a role in the onset of the disease.21
HLA-A29
HLA-A29 positivity is highly correlated with birdshot chorioretinopathy. This condition is characterized by oval, lightly-colored lesions that follow the vascular arcade in the retina. It can lead to progressive retinopathy, macular edema and epiretinal membranes. It is found highest in patients over 50 of Western European descent.
Serum Angiotensin-Converting Enzyme (ACE)
Angiotensin-converting enzyme is a cell membrane-bound protein involved in the conversion of angiotensin I to angiotensin II. Angiotensin II is part of a complex system responsible for regulating blood pressure and electrolytes. The sensitivity of ACE levels for detecting sarcoidosis is relatively low and can lead to false negatives. However, when elevated, it can be used to track treatment response after beginning immunotherapy. The conventional cut-off for the diagnosis of sarcoidosis is 21.4 IU/L, which has a sensitivity and specificity of 42% and 99%, respectively. A March 2023 retrospective study analyzed 3304 patient records and recommended adjusting the cut-off to ≥14.7. The new reference point changes the sensitivity to 78% and slightly decreases the specificity to 82%.24
Interferon Gamma (IFN-) Release Assay (IGRA) or QuantiFERON-TB Gold In-Tube (QFT) assay
The QFT assay is an enzyme-linked immunosorbent assay (ELISA) and is one of two types of IGRAs available to test for tuberculosis infection. It functions by using a sample of whole blood and measures the cell-mediated immune response when presented with antigens of M. tuberculosis (TB) against a control. It is unable to differentiate between active and latent TB infection.23
Antinuclear antibodies (ANA)
A lab test that indicates abnormally high levels of antinuclear antibodies. Elevated levels can indicate autoimmune conditions, such as Lupus. Lupus rarely presents with uveitis, so baseline testing of ANA is not recommended.12
Conclusion
This case highlights the importance of extensive history and testing when evaluating a patient with uveitis suspected of having systemic involvement. Uveitis can pose a clinical challenge due to the vast array of differential diagnoses. However, optometrists are well-positioned to decipher the nuances with attention to case history, recurrence patterns, anatomical location, and bilaterality. Acute unilateral non-granulomatous anterior uveitis is commonly idiopathic. Prompt aggressive treatment is recommended, but no further workup is usually necessary. If the patient presents with a granulomatous, bilateral, or recurrent uveitis, further investigation is needed with laboratory tests and imaging. Baseline testing, including CBC, CMP, syphilis serology, ESR, CRP and chest X-Ray, can be useful for determining the overall health of the patient. If sarcoidosis is suspected, ACE may help point to the diagnosis. However, keep in mind its variability and fair predictive value. Tests evaluating specific disease processes can be ordered judiciously when clinical examination, uveitis classification and differentials are considered.
References
- Sève P, Cacoub P, Bodaghi B, et al. Uveitis: Diagnostic work-up. A literature review and recommendations from an expert committee. Autoimmun Rev. 2017;16(12):1254-1264. doi:10.1016/j.autrev.2017.10.010
- de Parisot A, Kodjikian L, Errera MH, et al. Randomized Controlled Trial Evaluating a Standardized Strategy for Uveitis Etiologic Diagnosis (ULISSE). Am J Ophthalmol. 2017;178:176-185. doi:10.1016/j.ajo.2017.03.029
- Foster CS, Kothari S, Anesi SD, et al. The Ocular Immunology and Uveitis Foundation preferred practice patterns of uveitis management. Surv Ophthalmol. 2016;61(1):1-17. doi:10.1016/j.survophthal.2015.07.001
- Waseem S, Ahmed SH, Fatima S, Shaikh TG, Ahmed J. SARS-CoV-2 vaccination and uveitis: Are they linked? Ann Med Surg. 2022;81(June):104472. doi:10.1016/j.amsu.2022.104472
- Jabs DA, Nussenblatt RB, Rosenbaum JT, Group. S of UN (SUN) W. Standardization of uveitis nomenclature for reporting clinical data. Am J Ophthalmol. 2005;140(3):509-516. doi:10.1016/j.ajo.2005.03.057.Standardization
- Jakob E, Reuland MS, Mackensen F, et al. Uveitis subtypes in a German interdisciplinary uveitis center – Analysis of 1916 patients. J Rheumatol. 2009;36(1):127-136. doi:10.3899/jrheum.080102
- Tsirouki T, Dastiridou A, Symeonidis C, et al. A Focus on the Epidemiology of Uveitis. Ocul Immunol Inflamm. 2018;26(1):2-16. doi:10.1080/09273948.2016.1196713
- Tsirouki T, Dastiridou A, Symeonidis C, et al. A Focus on the Epidemiology of Uveitis. Ocul Immunol Inflamm. 2018;26(1):2-16. doi:10.1080/09273948.2016.1196713
- Curragh DS, McAvoy CE, Rooney M, McLoone E. Post-streptococcal uveitis syndrome in a Caucasian population: a case series. Eye. 2019;33(3):380-384. doi:10.1038/s41433-018-0214-0
- Becker MD, Jakob E, Mackensen F. Fuchs Uveitis Syndrome. Intraocular Inflamm. July 2022:955-965. doi:10.1007/978-3-540-75387-2_86
- Kuiper JJW, Venema WJ. HLA-A29 and Birdshot Uveitis: Further Down the Rabbit Hole. Front Immunol. 2020;11(November):1-14. doi:10.3389/fimmu.2020.599558
- McKay KM, Lim LL, Van Gelder RN. Rational laboratory testing in uveitis: A Bayesian analysis. Surv Ophthalmol. 2021;66(5):802-825. doi:10.1016/j.survophthal.2021.02.002
- Complete Blood Cell Count (CBC), MedLine Plus. https://medlineplus.gov/lab-tests/complete-blood-count-cbc/. Published March 16, 2023. Accessed March 16, 2023.
- Sweiss NJ, Salloum R, Ghandi S, et al. Significant CD4, CD8, and CD19 lymphopenia in the peripheral blood of sarcoidosis patients correlates with severe disease manifestations. PLoS One. 2010;5(2):3-6. doi:10.1371/journal.pone.0009088
- Majumder PD, Sudharshan S, Biswas J. Laboratory support in the diagnosis of uveitis. Indian J Ophthalmol. 2013;61(6):269-276. doi:10.4103/0301-4738.114095
- Comprehensive Metabolic Panel (CMP). Comprehensive Metabolic Panel (CMP).
- Satyaputra F, Hendry S, Braddick M, Sivabalan P, Norton R. The Laboratory Diagnosis of Syphilis. J Clin Microbiol. 2021;59(10):e0010021. doi:10.1128/JCM.00100-21
- Syphilis – STI Treatment Guidelines. https://www.cdc.gov/std/treatment-guidelines/syphilis.htm#nontreponemal-tests. Accessed March 16, 2023.
- Ebrahimiadib N, Berijani S, Ghahari M, Pahlaviani FG. Ankylosing spondylitis. J Ophthalmic Vis Res. 2021;16(3):462-469. doi:10.18502/jovr.v16i3.9440
- Skalska U, Kozakiewicz A, Mäliński W, Jurkowska M. HLA-B27 detection – Comparison of genetic sequence-based method and flow cytometry assay. Reumatologia. 2015;53(2):74-78. doi:10.5114/reum.2015.51506
- Takeno M. The association of Behçet’s syndrome with HLA-B51 as understood in 2021. Curr Opin Rheumatol. 2022;34(1):4-9. doi:10.1097/BOR.0000000000000846
- Coates D. The angiotensin converting enzyme (ACE). Int J Biochem Cell Biol. 2003;35(6):769-773. doi:10.1016/S1357-2725(02)00309-6
- Hu X, Zou L, Wang S, et al. Performance of Serum Angiotensin-Converting Enzyme in Diagnosing Sarcoidosis and Predicting the Active Status of Sarcoidosis: A Meta-Analysis. Biomolecules. 2022;12(10). doi:10.3390/biom12101400
- Kawai H, Hiroyuki Naruse, Sarai M, et al. Serum angiotensin‐converting enzyme levels indicating early sarcoidosis diagnosis and immunosuppressive therapy efficacy. 2023;10(3):1803-1810. doi:https://doi.org/10.1002/ehf2.14343
Jennifer Elder, OD, FAAO received her optometric education at the Southern College of Optometry in Memphis, TN. She went on to complete a residency at the Memphis VAMC. After residency, she was an associate at an ophthalmology/optometry practice in rural middle Tennessee. In 2020, she joined the Memphis VAMC at their community based outpatient clinic in Jackson, TN. She now resides in Jackson and spends her time outside of clinic with her husband and three young boys.