Principles of Diabetic Care

Abstract
Introduction: Ninety-five percent of diabetes cases are the hyperglycemic type 2 classification. This paper reviews pathophysiology principles in diabetes and surveys evidence-based protocols to improve outcomes in diabetic patients. In addition, diagnostic imaging in a case of proliferative diabetic retinopathy is presented.
Discussion: The Diabetes Prevention Program (DPP), United Kingdom Prospective Diabetic Study (UKPDS), and other important investigations provide the foundation for evidence-based recommendations to manage diabetes and lower the risk of complications. The most common diabetic complication, intraocular microvascular disease, is presented here with a case report and imaging.
Conclusion: Evidence shows that lifestyle modifications (LSM), well-timed systemic treatment, and ongoing counseling can prevent diabetes onset and lower the risk of disease complications in diabetic patients.
Keywords: Proliferative diabetic retinopathy, metabolic syndrome, diabetic macular edema, aflibercept, panretinal photocoagulation
Introduction
Type 2 diabetic pathophysiology originates from low pancreatic insulin production, resistance or ineffective action of produced insulin, and defects in insulin secretion related to inflammation or metabolic stress.1 It has been estimated that 13 percent of adults have diabetes worldwide.1 Ninety-five percent of diabetes is the hyperglycemic, type 2 classification1 associated with increasing age, obesity, and lack of physical exercise.2 The progression from early-stage insulin resistance, beginning as postprandial hyperglycemia, to the development of type 2 diabetes results from chronic hyperinsulinemia.3 Lifestyle modifications (LSM) and glycemic and blood pressure recommendations are established by study guidance to decrease systemic and ocular complications.
Discussion
Type 2 Diabetes Pathophysiology
Hyperinsulinemia is a pancreatic beta-cell response triggered to normalize glucose yet leads to hyperglycemia as impaired compensatory beta-cell mechanisms cause insulin resistance.2,3 The type 2 diabetes diagnostic glucose levels are based on their association with macro- and microvascular complication prevalence.1,2 The American Diabetic Association (ADA) criteria for diabetes diagnosis includes a glycosylated hemoglobin (A1C) test result greater than or equal to 6.5 percent, a fasting glucose level greater than or equal to 126mg/dL after no caloric intake for at least eight hours, a two-hour plasma glucose level of greater than or equal to 200 mg/dL using a glucose load containing 75-g anhydrous glucose dissolved in water, and a random plasma glucose level of greater than or equal to 200 mg/dL with hyperglycemic symptoms (polyuria, polydipsia, weight loss).2
Factors that influence the onset of diabetes include a combination of demographic, socio-environmental, ethnic origin, systemic vascular health, activity level, diet, general health habits, and first-degree family history.3 Physical inactivity is defined as less than ten minutes of exercise per week in potential activity areas of leisure time, work, and transportation.6 Body mass index greater than 25 kg/m2, blood pressure greater than or equal to 140/90 mmHg, high-density lipoprotein (HDL) level greater than or equal to 35 mg/dL, and triglyceride level greater than or equal to 250 mg/dL are also risk factors. Metabolic Syndrome (MetS) includes abdominal obesity, dyslipidemia, hypertension, and hyperglycemia, and the presence of this group of disorders creates a prime environment for the vascular complications of type 2 diabetic disease. Evidence suggests that specific diet modifications and a routine of physical activity are the most significant factors in reducing diabetes risk.4 Lifelong monitoring and culturally competent, structured self-management programs encourage long-term glycemic control.5
Relevant Studies
The Diabetes Prevention Program (DPP) randomized trial investigated the benefits of intensive lifestyle interventions through diet, physical activity, and insulin-sensitizing medications for diabetes risk reduction.1 Ten years later, the follow-up DPP Outcomes Study (DPPOS) results found that in high-risk individuals, a minimum of seven percent weight loss, 150 minutes of weekly physical activity, and dietary changes reduced diabetes onset rate by 34 percent, a shift from 58 percent seen in the initial study. The DPPOS also reported that medical treatment with metformin delayed or prevented diabetes onset by 18 percent, a change from 31 percent seen in the initial study.2
The United Kingdom Prospective Diabetes Study (UKPDS) determined that lowered microvascular disease risk was seen in subjects receiving intensive glucose therapy versus those relying on conventional dietary modifications.3 In addition, it found that tight control of blood pressure (<150/85 mmHg) reduced the risk of complications related to diabetes,4 including the deterioration of visual acuity. The UKPDS investigation determined that early and prolonged glucose control was most beneficial for limiting later complications, and the first few years after initial diagnosis were the most crucial.5,6
Further, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial results showed an unexpectedly increased risk of death from cardiovascular disease when the A1C goal was to maintain levels under 6 percent versus less strict ranges between 7-7.9 percent.7 Also, the results showed that the rate of fatal cardiovascular events was not reduced, targeting a systolic blood pressure of <120 mmHg, compared with <140 mmHg or with a combination of fenofibrate and simvastatin versus simvastatin alone.16 The ACCORD study’s importance is evident when eye care providers advise levels of glycemic control and blood pressure targets for persons with type 2 diabetes and high-risk cardiovascular disease profiles.
The ADA recommends statin therapy for persons with both diabetes and atherosclerotic cardiovascular disease (ASCVD).8 For persons under 40 years old with type 2 diabetes and no ASCVD risk factors, statin therapy is still recommended.17 Additionally, The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study showed that the lipid-lowering fibrate class of medications reduced the laser treatment rate for maculopathy and PDR compared to a statin alone.9 In combination, fenofibrate and statins showed a significant reduction in intraocular disease progression.18
The Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) study proved that the glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide was superior in glycated hemoglobin reduction and achieving weight loss goals.10 The benefit of weight loss for disease prevention is well studied, and there is recent data correlating the efficacy and durability of intermittent fasting programs with type 2 diabetes remission.11
Proliferative Diabetic Retinopathy and Macula Edema
Retinopathy with and without diabetic macular edema (DME) is the most common diabetic microvascular complication in the diabetic population.12,13 Hyperglycemic-induced intraocular inflammatory markers and oxidative stress are precursors for vascular permeability, vascular closure, and the hemodynamic alterations that effectuate new vessel growth in proliferative diabetic retinopathy (PDR).14 The extraretinal fibrovascular proliferation develops from myofibroblast and inflammatory cell migration and manifests as neovascularization on the optic nerve and elsewhere on the retinal surface.23 The diabetic macular edema (DME) nomenclature (center-involved or non-center-involved) distinguishes the presence of intraretinal fluid (IRF) or retinal thickening inside or outside the one-millimeter central subfield zone.23 The central retinal thickness and the central capillary integrity can be examined with OCT and OCT angiography imaging. The OCTA vitreoretinal interface (VRI) slab image can display neovascularization proliferating along the retinal surface.
Management
The merits of anti-vascular endothelial growth factor intravitreal injectable drugs have been studied extensively. While pan-retinal photocoagulation (PRP) has been the standard of care for patients with PDR, clinical studies show that the anti-VEGF agent, aflibercept used for OCT-evidenced center-involved DME and retinal neovascularization (NVE) is a beneficial alternative to PRP in cases of PDR15. The double-masked randomized prospective multicenter phase 2 DME And VEGF Trap-Eye Intravitreal Aflibercept Injection Investigation of Clinical Impact (DA VINCI) study assessed the efficacy of aflibercept, also known as VEGF trap eye (VTE), compared to standard-of-care laser treatment, and reported that aflibercept improved visual acuity when compared with macular laser photocoagulation.16
The Study of Intravitreal Aflibercept Injection in Patients with Diabetic Macular Edema (VISTA-DME) and the Intravitreal Aflibercept Injection in Vision Impairment Due to DME (VIVID-DME) studied the effect of intravitreal aflibercept on vision impairment due to DME and concluded that intravitreal aflibercept was associated with better anatomic outcomes and more gains in best-corrected visual acuity than macular laser treatment.17, The 148-week results of the VISTA and VIVID studies with 4-week and 8-week treatment interval groups confirmed that anatomic and visual improvements observed with intravitreal aflibercept injections over laser control were maintained at week 148 with similar efficacy.18
The double-masked, worldwide, multi-centered, 100-week, randomized Study of the Efficacy and Safety of Intravitreal Aflibercept for the Improvement of Moderately Severe to Severe Nonproliferative Diabetic Retinopathy (PANORAMA) clinical trial studied the efficacy of aflibercept to treat severe non-proliferative diabetic retinopathy (NPDR). PANORAMA found treatment with aflibercept resulted in fewer vision-threatening complications, including center-involved DME development. 19
RETINAL Photography and Imaging Case
In the following case, a series of retinal images and photographs show proliferative diabetic retinopathy resulting from poorly controlled diabetes in a 56-year-old male with an eight-year history of diabetes. At the time of presentation, his A1C was 9.3%. Hyperglycemic-induced intraocular inflammation and pro-angiogenic vascular endothelial growth factor (VEGF) caused severe retinal hypoxia and the resultant microvascular disease (see Table 1 for retinal findings).

Table 1. Dilated retinal exam findings
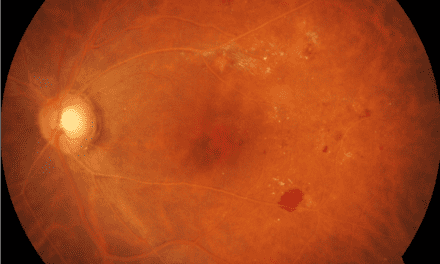
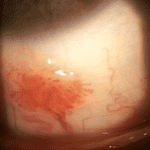
Figure 1 shows wide-field photography of the posterior pole in the right eye documenting four quadrants of dot and blot hemorrhages, a cotton wool spot, angiogenesis in the form of vein-to-vein anastomoses, and retinal neovascularization. Figure 2 shows four quadrants of intraretinal hemorrhages, venous beading, and a frond of neovascularization with early fibrotic traction in the inferior temporal retina of the left eye.

Figure 1. Wide field fundus photo of the right eye showing severe superior retinal hemorrhages and retinal neovascularization in proliferative diabetic retinopathy

Figure 2. Wide field fundus photo of fibrotic proliferation in the left eye after the first pan-retinal photocoagulation treatment/caption
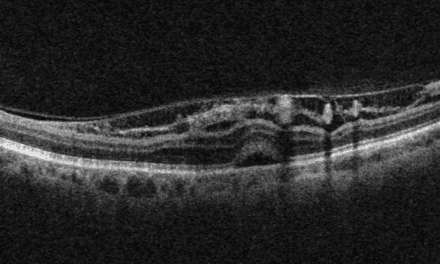
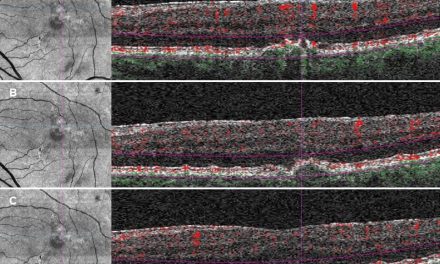
Optical coherence tomography (OCT) images of the left macula showed central inner and outer retinal cystoid edema captured on a one-line raster image (Figure 3). In addition, the 12×12 dimension OCT angiography (OCTA) imaged the large inferior temporal neovascular frond in the left eye, visible on the vitreoretinal interface (VRI) slab (Figure 4). Areas of neovascularization and severe capillary nonperfusion were evident with OCTA in both eyes (Figures 5-6).
Following two treatments of panretinal photocoagulation and three intravitreal aflibercept injections, the left eye developed inferior preretinal hemorrhages and vitreoretinal traction, despite the appearance of resolving center-involved macular edema and retinal neovascularization regression on OCTA (Figures 7-11).


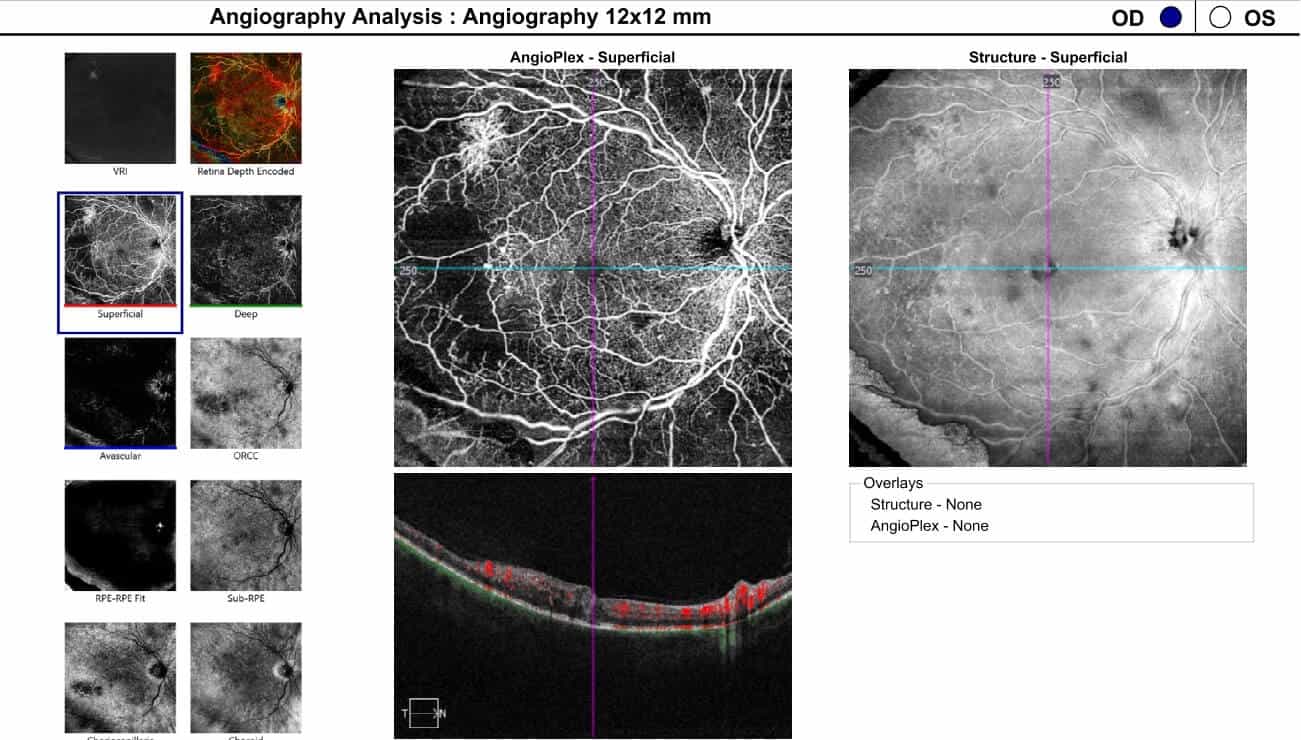


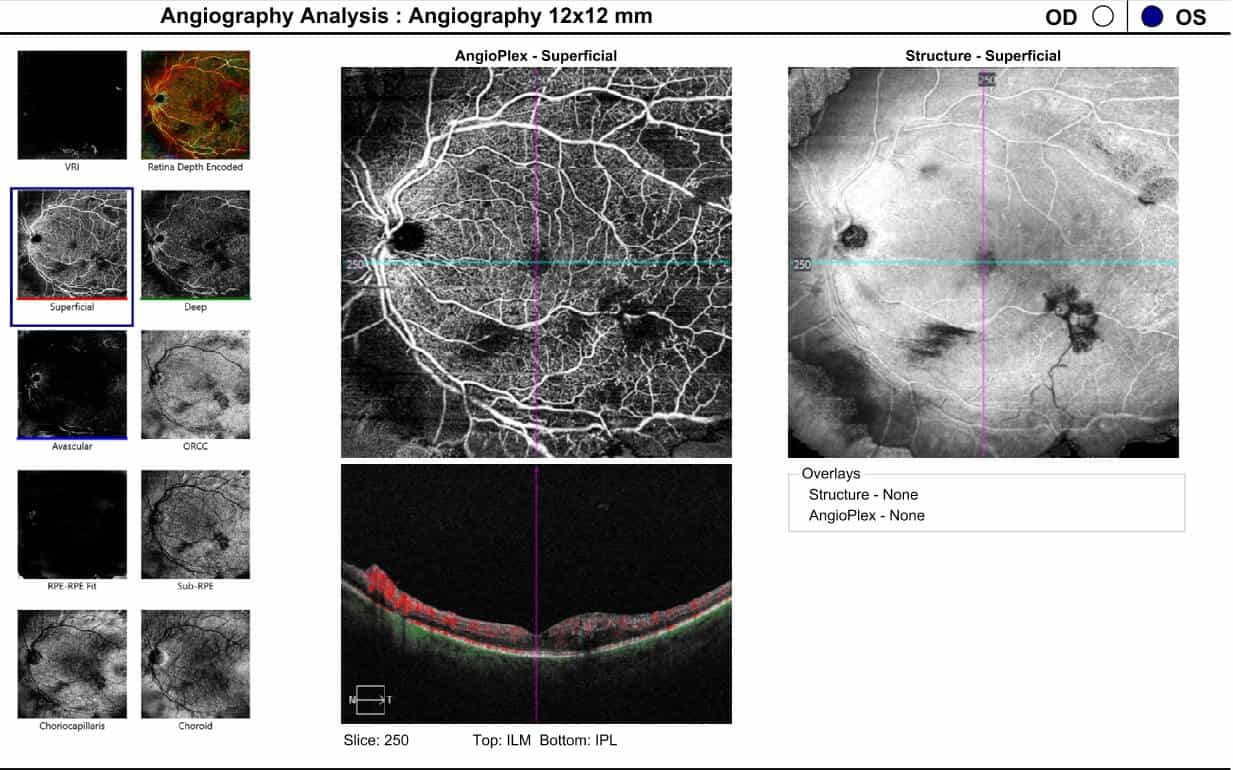



Diabetic counseling, including dietary and physical activity recommendations, was initiated at the initial optometry visit. The patient’s primary care provider added semaglutide to his regimen of insulin glargine human, antidiabetic agent empagliflozin prescribed to reduce renal glucose reabsorption, and anti-hypertensive losartan. Continuous dietary and LSM counseling resulted in the patient starting a daily walking exercise regimen, losing weight, and decreased his A1C to 7.4%.
Conclusion
The role of the eye care provider in diabetic care starts with prevention counseling and familiarization with systemic treatment plans to assure timely intervention for ocular disease. Moreover, collaboration with the medical team to institute the appropriate systemic treatment recommendations based on the current literature is essential to preventing vision loss.
References
[1] World Health Organization. Diabetes Facts Sheet, 2022. World Health Organization, https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed December 9, 2022
[2] American Diabetes Association; 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 1 January 2021; 44 (Supplement_1): S15–S33
[3] Ramlo-Halsted BA, Edelman SV. The natural history of type 2 diabetes. Implications for clinical practice. Primary Care 1999;26(4):771-89
[4] Riddle MC, Hetzel C, Gerstein PD. Lingering effects of hyperglycemia in recently diagnosed diabetes during long-term follow-up of the DCCT/EDIC and UKPDS Cohorts: more evidence that early control matters. Diabetes Care 2021;44(10):2212–2215
[5] Holman RR, Paul SR, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Eng J Med 2008;359(15):1577-98
[6] Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 9, 2022
[7] Rietz M, Lehr A, Mino E, Lang A, et al. Physical activity, and risk of major diabetes-related complications in individuals with diabetes: a systematic review and meta-analysis of observational studies. Diabetes Care 1 2022; 45(12): 3101–3111
[8] Scaub SF, Friedman R, Gross JL. A structural educational program in patients with type 2 diabetes: A randomized controlled trial. Diabetes Educ 2009; 35(4):603-11
[9] Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. 2002. Diab Care 25:2165-2171
[10] White NH, Pan Q, Knowler WC, et al. Diabetes Prevention Program Outcome Study (DPPOS) Research Group. Risk Factors for the development of retinopathy in prediabetes and type 2 diabetes: The Diabetic Prevention Program Experience. Diabetes Care. 2022;45(11):2653-2661
[11] Holman RR, Paul SR, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Eng J Med 2008;359(15):1577-98
[12] Matthews DR, Stratton IM, Aldington SJ, et al. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS69. Arch Ophthalmol 2004; 122(11):1631-40
[13] Lind M, Imberg H, Coleman. RL, et al. Historical HbA1c Values may explain the type 2 diabetes legacy effect: UKPDS 88, Diabetes Care 2021;44:2231-2237
[14] Riddle MC, Hertzel C, Gerstein PD. Lingering effects of hyperglycemia in recently diagnosed diabetes during long-term follow-up of the DCCT/EDIC and UKPDS Cohorts: more evidence that early control matters. Diabetes Care 2021;44(10):2212–2215
[15] ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood pressure control in type 2 diabetes Mellitus. N Engl J Med 2010;362(17):1575-85
[16] American Diabetes Association. Standards of medical care in diabetes – 2019. Diabetic Care 2019;42(Supple):S-1183
[17] Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD Study): a randomised controlled trial. Lancet 2007;370(9600):1687-97
[18] Goldenberg RM, Steen O. Semaglutide: Review and place in therapy for adults with type 2 diabetes. Can J Diabetes. 2019 Mar;43(2):136-145
[19] Yang X, Zhou J, Shao H, et al. Effect of an Intermittent Calorie-restricted Diet on Type 2 Diabetes Remission: A Randomized Controlled Trial. J Clin Endocrinol Metab. 2022 Dec 14:dgac661
[20] Miller K, Fortun JA. Diabetic Macular Edema: Current understanding, pharmacologic treatment options, and developing therapies. Asia Pac J Ophthalmol (Phila). 2018;7(1):28-35
[21] Hussain, R.M.; Neiweem, A.E.; Kansara, V.; Harris, A.; Ciulla, T.A. Tie-2/Angiopoietin pathway modulation as a therapeutic strategy for retinal disease. Expert Opin Investig Drugs 2019, 28, 861–869
[22] American Optometric Association. (2019). Evidence-Based Clinical Guidelines: Eyecare of the patient with diabetes. St Louis, MO
[23] Sivaprasad S, Hykin P, Prevost AT et al. Intravitreal aflibercept compared with panretinal photocoagulation for proliferative diabetic retinopathy: the CLARITY non-inferiority RCT. Efficacy Mech Eval 2018:5(5)
[24] Do DV, Nguyen QD, Boyer D, et al. da Vinci Study Group. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012 Aug;119(8):1658-65
[25] Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 2015;122(10)
[26] Heier JS, Korobelnic J, Brown D, et al. Intravitreal Aflibercept for Diabetic Macular Edema148-Week Results from the VISTA and VIVID Studies. Ophthalmology 2016;123(11):2376-2385
[27] Brown DM, Wykoff CC, Boyer D, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: Results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;139(9):946–95
Leslie Wilderson, OD, FAAO, Dipl. (CEC), attended the Nova Southeastern University School of Optometry, completed a residency in primary care, and is board-certified in medical optometry. She is a member of the Northeast Ohio VA Hospital clinical optometry staff and a United States Air Force Reserve Officer.